Apo2L/TRAIL inhibits tumor growth and bone destruction in a murine model of multiple myeloma
- PMID: 19276263
- PMCID: PMC5573683
- DOI: 10.1158/1078-0432.CCR-08-2444
Apo2L/TRAIL inhibits tumor growth and bone destruction in a murine model of multiple myeloma
Abstract
Purpose: Multiple myeloma is an incurable disease, for which the development of new therapeutic approaches is required. Here, we report on the efficacy of recombinant soluble Apo2L/tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to inhibit tumor progression and bone destruction in a xenogeneic model of human multiple myeloma.
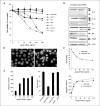
Experimental design: We established a mouse model of myeloma, in which Apo2L/TRAIL-sensitive RPMI-8226 or KMS-11 cells, tagged with a triple reporter gene construct (NES-HSV-TK/GFP/Luc), were transplanted directly into the tibial marrow cavity of nude mice. Tumor burden was monitored progressively by bioluminescence imaging and the development of myeloma-induced osteolysis was measured using high resolution in vivo micro-computed tomography.
Results: Tumor burden increased progressively in the tibial marrow cavity of mice transplanted with Apo2L/TRAIL-sensitive RPMI-8226 or KMS-11 cells associated with extensive osteolysis directly in the area of cancer cell transplantation. Treatment of mice with recombinant soluble Apo2L/TRAIL reduced myeloma burden in the bone marrow cavity and significantly protected against myeloma-induced osteolysis. The protective effects of Apo2L/TRAIL treatment on bone were mediated by the direct apoptotic actions of Apo2L/TRAIL on myeloma cells within the bone microenvironment.
Conclusions: This is the first in vivo study that investigates the efficacy of recombinant Apo2L/TRAIL on myeloma burden within the bone microenvironment and associated myeloma-induced bone destruction. Our findings that recombinant soluble Apo2L/TRAIL reduces myeloma burden within the bone microenvironment and protects the bone from myeloma-induced bone destruction argue against an inhibitory role of osteoprotegerin in Apo2L/TRAIL-induced apoptosis in vivo and highlight the need to clinically evaluate Apo2L/TRAIL in patients with multiple myeloma.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




Similar articles
-
Apo2l/Tumor necrosis factor-related apoptosis-inducing ligand prevents breast cancer-induced bone destruction in a mouse model.Cancer Res. 2006 May 15;66(10):5363-70. doi: 10.1158/0008-5472.CAN-05-4386. Cancer Res. 2006. PMID: 16707463
-
Anticancer efficacy of Apo2L/TRAIL is retained in the presence of high and biologically active concentrations of osteoprotegerin in vivo.J Bone Miner Res. 2011 Mar;26(3):630-43. doi: 10.1002/jbmr.244. J Bone Miner Res. 2011. PMID: 20818644 Free PMC article.
-
Bone marrow stroma confers resistance to Apo2 ligand/TRAIL in multiple myeloma in part by regulating c-FLIP.J Immunol. 2008 Feb 1;180(3):1545-55. doi: 10.4049/jimmunol.180.3.1545. J Immunol. 2008. PMID: 18209050
-
Targeting Apo2L/TRAIL receptors by soluble Apo2L/TRAIL.Cancer Lett. 2013 May 28;332(2):156-62. doi: 10.1016/j.canlet.2010.11.001. Epub 2011 Jan 8. Cancer Lett. 2013. PMID: 21220186 Review.
-
The potential of the tumor microenvironment to influence Apo2L/TRAIL induced apoptosis.Immunol Invest. 2006;35(3-4):279-96. doi: 10.1080/08820130600745463. Immunol Invest. 2006. PMID: 16916755 Review.
Cited by
-
PTHrP Overexpression Increases Sensitivity of Breast Cancer Cells to Apo2L/TRAIL.PLoS One. 2013 Jun 18;8(6):e66343. doi: 10.1371/journal.pone.0066343. Print 2013. PLoS One. 2013. PMID: 23822995 Free PMC article.
-
Targeted Therapy With Immunoconjugates for Multiple Myeloma.Front Immunol. 2020 Jun 19;11:1155. doi: 10.3389/fimmu.2020.01155. eCollection 2020. Front Immunol. 2020. PMID: 32636838 Free PMC article. Review.
-
HDAC inhibition synergistically enhances alkylator-induced DNA damage responses and apoptosis in multiple myeloma cells.Cancer Lett. 2010 Oct 28;296(2):233-40. doi: 10.1016/j.canlet.2010.04.014. Epub 2010 May 5. Cancer Lett. 2010. PMID: 20447761 Free PMC article.
-
Apomab, a fully human agonistic antibody to DR5, exhibits potent antitumor activity against primary and metastatic breast cancer.Mol Cancer Ther. 2009 Oct;8(10):2969-80. doi: 10.1158/1535-7163.MCT-09-0745. Epub 2009 Oct 6. Mol Cancer Ther. 2009. PMID: 19808976 Free PMC article.
-
NS-018 reduces myeloma cell proliferation and suppresses osteolysis through inhibition of the JAK2 and Src signaling pathways.Blood Cancer J. 2018 Jun 25;8(7):62. doi: 10.1038/s41408-018-0098-z. Blood Cancer J. 2018. PMID: 29941953 Free PMC article. No abstract available.
References
-
- Croucher PI, Apperley JF. Bone disease in multiple myeloma. Br J Haematol. 1998;103:902–10. - PubMed
-
- Zannettino AC, Farrugia AN, Kortesidis A, et al. Elevated serum levels of stromal-derived factor-1 α are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005;65:1700–9. - PubMed
-
- Farrugia AN, Atkins GJ, To LB, et al. Receptor activator of nuclear factor-кB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003;63:5438–45. - PubMed
-
- Heider U, Hofbauer LC, Zavrski I, Kaiser M, Jakob C, Sezer O. Novel aspects of osteoclast activation and osteoblast inhibition in myeloma bone disease. Biochem Biophys Res Commun. 2005;338:687–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

