The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion
- PMID: 19276380
- PMCID: PMC3718046
- DOI: 10.1158/0008-5472.CAN-08-2394
The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion
Abstract
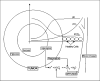
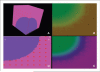
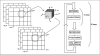
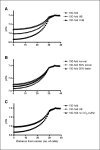
A number of studies have shown that the extracellular pH (pHe) in cancers is typically lower than that in normal tissue and that an acidic pHe promotes invasive tumor growth in primary and metastatic cancers. Here, we investigate the hypothesis that increased systemic concentrations of pH buffers reduce intratumoral and peritumoral acidosis and, as a result, inhibit malignant growth. Computer simulations are used to quantify the ability of systemic pH buffers to increase the acidic pHe of tumors in vivo and investigate the chemical specifications of an optimal buffer for such purpose. We show that increased serum concentrations of the sodium bicarbonate (NaHCO(3)) can be achieved by ingesting amounts that have been used in published clinical trials. Furthermore, we find that consequent reduction of tumor acid concentrations significantly reduces tumor growth and invasion without altering the pH of blood or normal tissues. The simulations also show that the critical parameter governing buffer effectiveness is its pK(a). This indicates that NaHCO(3), with a pK(a) of 6.1, is not an ideal intratumoral buffer and that greater intratumoral pHe changes could be obtained using a buffer with a pK(a) of approximately 7. The simulations support the hypothesis that systemic pH buffers can be used to increase the tumor pHe and inhibit tumor invasion.
Figures
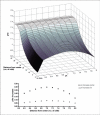
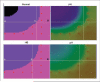
References
-
- Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 2004;23:57–64. - PubMed
-
- Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging. 2002;16:430–50. - PubMed
-
- Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–82. - PubMed
-
- Gallagher FA, Kettunen MI, Day SE, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453:940–3. - PubMed
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

