Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context
- PMID: 19276453
- PMCID: PMC2720697
- DOI: 10.1093/jnci/djp001
Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context
Abstract
Background: The time by which prostate-specific antigen (PSA) screening advances prostate cancer diagnosis, called the lead time, has been reported by several studies, but results have varied widely, with mean lead times ranging from 3 to 12 years. A quantity that is closely linked with the lead time is the overdiagnosis frequency, which is the fraction of screen-detected cancers that would not have been diagnosed in the absence of screening. Reported overdiagnosis estimates have also been variable, ranging from 25% to greater than 80% of screen-detected cancers.
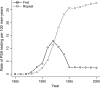
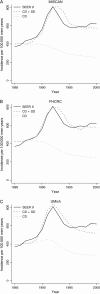
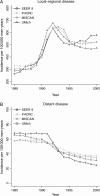
Methods: We used three independently developed mathematical models of prostate cancer progression and detection that were calibrated to incidence data from the Surveillance, Epidemiology, and End Results program to estimate lead times and the fraction of overdiagnosed cancers due to PSA screening among US men aged 54-80 years in 1985-2000. Lead times were estimated by use of three definitions. We also compared US and earlier estimates from the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer (ERSPC) that were calculated by use of a microsimulation screening analysis (MISCAN) model.
Results: The models yielded similar estimates for each definition of lead time, but estimates differed across definitions. Among screen-detected cancers that would have been diagnosed in the patients' lifetimes, the estimated mean lead time ranged from 5.4 to 6.9 years across models, and overdiagnosis ranged from 23% to 42% of all screen-detected cancers. The original MISCAN model fitted to ERSPC Rotterdam data predicted a mean lead time of 7.9 years and an overdiagnosis estimate of 66%; in the model that was calibrated to the US data, these were 6.9 years and 42%, respectively.
Conclusion: The precise definition and the population used to estimate lead time and overdiagnosis can be important drivers of study results and should be clearly specified.
Figures


Comment in
-
Why are a high overdiagnosis probability and a long lead time for prostate cancer screening so important?J Natl Cancer Inst. 2009 Mar 18;101(6):362-3. doi: 10.1093/jnci/djp028. Epub 2009 Mar 10. J Natl Cancer Inst. 2009. PMID: 19276451 No abstract available.
References
-
- De Koning HJ, Liem MK, Baan CA, Boer R, Schroder FH, Alexander FE. Prostate cancer mortality reduction by screening: power and time frame with complete enrollment in the European Randomised Screening for Prostate Cancer (ERSPC) trial. Int J Cancer. 2002;98(2):268–273. - PubMed
-
- Gohagan JK, Prorok PC, Kramer BS, Cornett JE. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial of the National Cancer Institute [see comments] J Urol. 1994;152(5 pt 2):1905–1909. - PubMed
-
- Oliver SE, May MT, Gunnell D. International trends in prostate-cancer mortality in the “PSA era”. Int J Cancer. 2001;92(6):893–898. - PubMed
-
- Korfage IJ, Essink-Bot ML, Borsboom GJ, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int J Cancer. 2005;116(2):291–296. - PubMed
-
- Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273(4):289–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

