Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus
- PMID: 19276628
- PMCID: PMC2904473
- DOI: 10.1159/000208211
Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus
Abstract
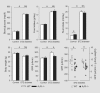
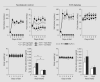
Background: In early type 1 diabetes mellitus, changes in proximal reabsorption influence glomerular filtration rate (GFR) through tubuloglomerular feedback (TGF). Due to TGF, a primary increase in proximal reabsorption causes early diabetic hyperfiltration, while a heightened sensitivity of the proximal tubule to dietary salt leads to the so-called salt paradox, where a change in dietary salt causes a reciprocal change in GFR ('tubulocentric principle'). Here, experiments were performed in adenosine A(1) receptor knockout mice (A(1)R-/-), which lack an immediate TGF response, to determine whether A(1)Rs are essential for early diabetic hyperfiltration and the salt paradox.
Methods: GFR was measured by inulin disappearance in conscious A(1)R-/- and wild-type (WT) mice after 4 weeks of streptozotocin diabetes on a control NaCl diet (1%), and measurements were repeated after 6 days of equilibration on a low-NaCl (0.1%) or a high-NaCl (4%) diet.
Results: A(1)R-/- and WT were similar with respect to blood glucose, dietary intakes and body weight changes on a given diet. Diabetic hyperfiltration occurred in WT, but was blunted in A(1)R-/-. A reciprocal relationship between GFR and dietary salt was found in WT diabetics, but not A(1)R-/- diabetics or nondiabetics of either strain.
Conclusion: A(1)Rs determine glomerular hyperfiltration and the salt paradox in early diabetes, which is consistent with the tubulocentric principle.
Figures

References
-
- Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol. 2003;14:530–537. - PubMed
-
- Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. - PubMed
-
- Mogensen CE. Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest. 1986;46:201–206. - PubMed
-
- Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

