F- and G-actin concentrations in lamellipodia of moving cells
- PMID: 19277198
- PMCID: PMC2652108
- DOI: 10.1371/journal.pone.0004810
F- and G-actin concentrations in lamellipodia of moving cells
Abstract
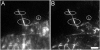
Cells protrude by polymerizing monomeric (G) into polymeric (F) actin at the tip of the lamellipodium. Actin filaments are depolymerized towards the rear of the lamellipodium in a treadmilling process, thereby supplementing a G-actin pool for a new round of polymerization. In this scenario the concentrations of F- and G-actin are principal parameters, but have hitherto not been directly determined. By comparing fluorescence intensities of bleached and unbleached regions of lamellipodia in B16-F1 mouse melanoma cells expressing EGFP-actin, before and after extraction with Triton X-100, we show that the ratio of F- to G-actin is 3.2+/-0.9. Using electron microscopy to determine the F-actin content, this ratio translates into F- and G-actin concentrations in lamellipodia of approximately 500 microM and 150 microM, respectively. The excess of G-actin, at several orders of magnitude above the critical concentrations at filament ends indicates that the polymerization rate is not limited by diffusion and is tightly controlled by polymerization/depolymerization modulators.
Conflict of interest statement
Figures

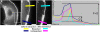
References
-
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res. 1970;59:393–398. - PubMed
-
- Small JV, Isenberg G, Celis JE. Polarity of actin at the leading edge of cultured cells. Nature. 1978;272:638–639. - PubMed
-
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976;108:139–150. - PubMed
-
- Waterman-Storer CM, Desai A, Bulinski JC, Salmon ED. Fluorescent speckle microscopy, a method to visualize the dynamics of protein assemblies in living cells. Curr Biol. 1998;8:1227–1230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

