Higher irradiance and photodynamic therapy for age-related macular degeneration (an AOS thesis)
- PMID: 19277246
- PMCID: PMC2646424
Higher irradiance and photodynamic therapy for age-related macular degeneration (an AOS thesis)
Abstract
Purpose: Photodynamic therapy (PDT) using verteporfin was the first pharmacologic therapy for neovascular age-related macular degeneration and changed the treatment paradigm for a major, blinding disease. The experimental work in the nonhuman primate was essential in developing treatment parameters for verteporfin PDT that could successfully occlude choroidal neovascularization with limited injury to the neural retina. Early in the preclinical primate studies, we hypothesized that higher irradiances could be used for ocular PDT than had been used in dermatology and other applications, which typically utilized an irradiance of 150 to 200 mW/cm(2). We set out to test the feasibility of irradiances up to 1800 mW/cm(2).
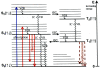
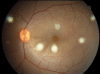
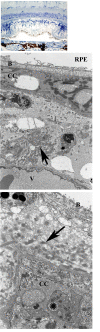
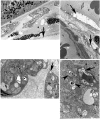
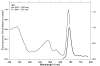
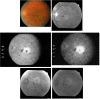
Methods: PDT was applied to normal monkey eyes using verteporfin/benzoporphyrin derivative (BPD) (2 mg/kg) mixed with low-density lipoprotein in DMSO, and 692-nm light, with a spot size 1250mum, fluence approximately 50 J/cm(2), and irradiance varying from 150 (treatment time, 6 minutes) to 1800 mW/cm(2) (treatment time, 30 seconds). Photocoagulation lesions were applied using 514-nm and 692-nm laser light without drug, with irradiance of 18,750 to 200,000 mW/cm(2) and spot size of 500 mum. Treatment effect was evaluated by fundus photography, angiography, and light and electron microscopy with collagen denaturation as a marker of thermal injury.
Results: Verteporfin/BPD PDT at irradiances of 150 to 1800 mW/cm(2) showed no collagen denaturation in contrast to photocoagulation lesions without dye (irradiance 10-fold and higher).
Conclusions: Verteporfin PDT could safely be performed at higher irradiances, permitting a clinically practical therapy. Ultimately, clinical trials demonstrated that verteporfin PDT could limit moderate vision loss in neovascular age-related macular degeneration. Although anti-VEGF therapy has replaced PDT as a first-line therapy, PDT may still have a role, perhaps in combination therapies. Further investigations to optimize drug delivery and to better understand the molecular mechanisms of PDT effects in both choroidal neovascularization and retina will improve its application in macular diseases.
Figures







Similar articles
-
Effects of photodynamic therapy using verteporfin on experimental choroidal neovascularization and normal retina and choroid up to 7 weeks after treatment.Invest Ophthalmol Vis Sci. 1999 Sep;40(10):2322-31. Invest Ophthalmol Vis Sci. 1999. PMID: 10476799
-
Comparison of reduced and standard light application in photodynamic therapy of the eye in two rabbit models.Graefes Arch Clin Exp Ophthalmol. 2006 Jul;244(7):773-81. doi: 10.1007/s00417-005-0221-2. Epub 2006 Jan 27. Graefes Arch Clin Exp Ophthalmol. 2006. PMID: 16440207
-
Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2.Am J Ophthalmol. 2001 May;131(5):541-60. doi: 10.1016/s0002-9394(01)00967-9. Am J Ophthalmol. 2001. PMID: 11336929 Clinical Trial.
-
Photodynamic therapy with verteporfin: a new treatment in ophthalmology.Semin Ophthalmol. 2001 Dec;16(4):201-6. doi: 10.1076/soph.16.4.201.10298. Semin Ophthalmol. 2001. PMID: 15513441 Review.
-
Clinical evidence of intravitreal triamcinolone acetonide in the management of age-related macular degeneration.Curr Drug Targets. 2011 Feb;12(2):149-72. doi: 10.2174/138945011794182746. Curr Drug Targets. 2011. PMID: 20887246 Review.
Cited by
-
Targeting the YAP/TAZ Pathway in Uveal and Conjunctival Melanoma With Verteporfin.Invest Ophthalmol Vis Sci. 2021 Apr 1;62(4):3. doi: 10.1167/iovs.62.4.3. Invest Ophthalmol Vis Sci. 2021. PMID: 33798262 Free PMC article.
-
Genetic predictors of response to photodynamictherapy.Mol Diagn Ther. 2011 Aug 1;15(4):195-210. doi: 10.1007/BF03256411. Mol Diagn Ther. 2011. PMID: 21913742 Review.
-
Verteporfin-induced formation of protein cross-linked oligomers and high molecular weight complexes is mediated by light and leads to cell toxicity.Sci Rep. 2017 Apr 21;7:46581. doi: 10.1038/srep46581. Sci Rep. 2017. Retraction in: Sci Rep. 2024 May 14;14(1):11040. doi: 10.1038/s41598-024-61893-8. PMID: 28429726 Free PMC article. Retracted.
-
The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment.Eye (Lond). 2011 Feb;25(2):127-39. doi: 10.1038/eye.2010.196. Epub 2010 Dec 24. Eye (Lond). 2011. PMID: 21183941 Free PMC article.
-
Intra-tumoral YAP and TAZ heterogeneity drives collective NSCLC invasion that is targeted by SUMOylation inhibitor TAK-981.iScience. 2024 Oct 11;27(11):111133. doi: 10.1016/j.isci.2024.111133. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524367 Free PMC article.
References
-
- Nanda S, Hatchell D, Tiedeman J, Dutton J, Hatchell M, McAdoo T. A new method of vascular occlusion. Photochemical initiation of thrombosis. Arch Ophthalmol. 1987;105:1121–1124. - PubMed
-
- Miller JW, Stinson WG, Gregory WA, El-Khoumy HA, Puliafito CA. Phthalocyanine photodynamic therapy of experimental iris neovascularization. Ophthalmology. 1991;98:1711–1719. - PubMed
-
- Kliman G, Puliafito C, Stern D, Borirakchanyavat S, Gregory W. Phthaloycyanine photodynamic therapy: new strategy for closure of choroidal neovascularization. Lasers Surg Med. 1994;15:2–10. - PubMed
-
- Kliman G, Puliafito C, Grossman G, Gregory W. Retinal and choroidal vessel closure using phthalocyanine photodynamic therapy. Lasers Surg Med. 1994;15:11–18. - PubMed
-
- Miller H, Miller B. Photodynamic therapy of subretinal neovascularization in the monkey eye. Arch Ophthalmol. 1993;111:855–860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
