Spectral domain optical coherence tomography for glaucoma (an AOS thesis)
- PMID: 19277249
- PMCID: PMC2646438
Spectral domain optical coherence tomography for glaucoma (an AOS thesis)
Abstract
Purpose: Optical coherence tomography (OCT) is a rapidly evolving, robust technology that has profoundly changed the practice of ophthalmology. Spectral domain OCT (SD-OCT) increases axial resolution 2- to 3-fold and scan speed 60- to 110-fold vs time domain OCT (TD-OCT). SD-OCT enables novel scanning, denser sampling, and 3-dimensional imaging. This thesis tests my hypothesis that SD-OCT improves reproducibility, sensitivity, and specificity for glaucoma detection.
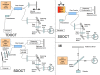
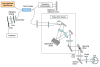
Methods: OCT progress is reviewed from invention onward, and future development is discussed. To test the hypothesis, TD-OCT and SD-OCT reproducibility and glaucoma discrimination are evaluated. Forty-one eyes of 21 subjects (SD-OCT) and 21 eyes of 21 subjects (TD-OCT) are studied to test retinal nerve fiber layer (RNFL) thickness measurement reproducibility. Forty eyes of 20 subjects (SD-OCT) and 21 eyes of 21 subjects (TD-OCT) are investigated to test macular parameter reproducibility. For both TD-OCT and SD-OCT, 83 eyes of 83 subjects are assessed to evaluate RNFL thickness and 74 eyes of 74 subjects to evaluate macular glaucoma discrimination.
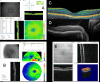
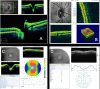
Results: Compared to conventional TD-OCT, SD-OCT had statistically significantly better reproducibility in most sectoral macular thickness and peripapillary RNFL sectoral measurements. There was no statistically significant difference in overall mean macular or RNFL reproducibility, or between TD-OCT and SD-OCT glaucoma discrimination. Surprisingly, TD-OCT macular RNFL thickness showed glaucoma discrimination superior to SD-OCT.
Conclusions: At its current development state, SD-OCT shows better reproducibility than TD-OCT, but glaucoma discrimination is similar for TD-OCT and SD-OCT. Technological improvements are likely to enhance SD-OCT reproducibility, sensitivity, specificity, and utility, but these will require additional development.
Figures






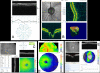



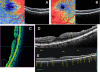
References
-
- de Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28:2067–2069. - PubMed
-
- Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121:695–706. - PubMed
-
- Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21:1361–1367. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
