Off-label, off-limits? Parental awareness and attitudes towards off-label use in paediatrics
- PMID: 19277709
- PMCID: PMC2772947
- DOI: 10.1007/s00431-009-0956-6
Off-label, off-limits? Parental awareness and attitudes towards off-label use in paediatrics
Abstract
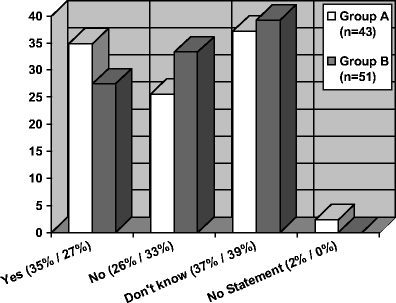
Off-label drug use in paediatrics is associated with an increased risk of adverse drug reactions. Any risk-benefit analysis has to be based on value judgments that should include parents' views. However, nothing is known so far about the parents' perspective on this critical issue. Therefore, a quantitative survey with parents of healthy and chronically ill children was carried out (n = 94). Knowledge about the practise of off-label use is generally poor in both groups. Surprisingly, this is also true for the parents of children with chronic disease. Nine percent of the parents of chronically ill children and 20% of the parents of healthy children would refuse treatment with an off-label drug. Parents who have poor knowledge about the practise of off-label use tend to refuse to volunteer their child for study participation. Therefore, the information of parents on the off-label use of drugs is important to meet ethical standards and to increase the parents' acceptance of medical studies with children.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources

