Aliphatic peptidyl hydroperoxides as a source of secondary oxidation in hydroxyl radical protein footprinting
- PMID: 19278868
- PMCID: PMC2684652
- DOI: 10.1016/j.jasms.2009.02.004
Aliphatic peptidyl hydroperoxides as a source of secondary oxidation in hydroxyl radical protein footprinting
Abstract
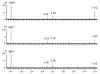
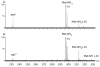
Hydroxyl radical footprinting is a technique for studying protein structure and binding that entails oxidizing a protein system of interest with diffusing hydroxyl radicals, and then measuring the amount of oxidation of each amino acid. One important issue in hydroxyl radical footprinting is limiting amino acid oxidation by secondary oxidants to prevent uncontrolled oxidation, which can cause amino acids to appear more solvent accessible than they really are. Previous work suggested that hydrogen peroxide was the major secondary oxidant of concern in hydroxyl radical footprinting experiments; however, even after elimination of all hydrogen peroxide, some secondary oxidation was still detected. Evidence is presented for the formation of peptidyl hydroperoxides as the most abundant product upon oxidation of aliphatic amino acids. Both reverse phase liquid chromatography and catalase treatment were shown to be ineffective at eliminating peptidyl hydroperoxides. The ability of these peptidyl hydroperoxides to directly oxidize methionine is demonstrated, suggesting the value of methionine amide as an in situ protectant. Hydroxyl radical footprinting protocols require the use of an organic sulfide or similar peroxide scavenger in addition to removal of hydrogen peroxide to successfully eradicate all secondary oxidizing species and prevent uncontrolled oxidation of sulfur-containing residues.
Figures
References
-
- Xu G, Chance M. Hydroxyl Radical-Mediated Modification of Proteins as Probes for Structural Proteomics. Chem Rev. 2007:3514–3543. - PubMed
-
- Konermann L, Tong X, Pan Y. Protein Structure and Dynamics Studied by Mass Spectrometry: H/D Exchange, Hydroxyl Radical Labeling, and Related Approaches. J Mass Spectrom. 2008;43:1021–36. - PubMed
-
- Chance MR. Unfolding of Apomyoglobin Examined by Synchrotron Footprinting. Biochem Biophys Res Commun. 2001;287:614–621. - PubMed
-
- Kiselar JG, Maleknia SD, Sullivan M, Downard KM, Chance MR. Hydroxyl Radical Probe of Protein Surfaces Using Synchrotron X-Ray Radiolysis and Mass Spectrometry. Int J Radiat Biol. 2002;78:101–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

