Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial
- PMID: 19278902
- PMCID: PMC2660392
- DOI: 10.1016/S1474-4422(09)70049-3
Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial
Abstract
Background: The propagation of prions, the causative agents of Creutzfeldt-Jakob disease and other human prion diseases, requires post-translational conversion of normal cellular prion protein to disease-associated forms. The antimalarial drug quinacrine (mepacrine) prevents this conversion in vitro, and was given to patients with various prion diseases to assess its safety and efficacy in changing the course of these invariably fatal and untreatable diseases.
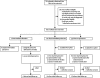
Methods: Patients with prion disease were recruited via the UK national referral system and were offered a choice between quinacrine (300 mg daily), no quinacrine, or randomisation to immediate quinacrine or deferred quinacrine in an open-label, patient-preference trial. The primary endpoints were death and serious adverse events possibly or probably related to the study drug. This study is registered, ISRCTN 06722585.
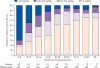
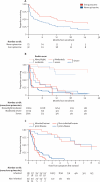
Findings: 107 patients with prion disease (45 sporadic, two iatrogenic, 18 variant, and 42 inherited) were enrolled, 23 in a pilot study and 84 in the main study. Only two patients chose randomisation; 40 took quinacrine during follow-up (37 who chose it at enrollment). Choice of treatment was associated with disease severity, with those least and most severely affected more likely to choose not to receive quinacrine. 78 (73%) patients died: one randomly assigned to deferred treatment, 26 of 38 who chose immediate quinacrine, and 51 of 68 who chose no quinacrine. Although adjusted mortality was lower in those who chose to take quinacrine than in those who did not, this was due to confounding with disease severity, and there was no difference in mortality between groups after adjustment. Four of 40 patients who took quinacrine had a transient response on neurological rating scales. Only two of 14 reported serious adverse events were judged quinacrine-related.
Interpretation: Quinacrine at a dose of 300 mg per day was reasonably tolerated but did not significantly affect the clinical course of prion diseases in this observational study.
Figures
Comment in
-
Clinical trials for prion disease: difficult challenges, but hope for the future.Lancet Neurol. 2009 Apr;8(4):304-6. doi: 10.1016/S1474-4422(09)70050-X. Epub 2009 Mar 9. Lancet Neurol. 2009. PMID: 19278901 Free PMC article. No abstract available.
-
Clinical trials and methodological problems in prion diseases.Lancet Neurol. 2009 Sep;8(9):782; author reply 782-3. doi: 10.1016/S1474-4422(09)70214-5. Lancet Neurol. 2009. PMID: 19679270 No abstract available.
References
-
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. - PubMed
-
- Wroe SJ, Pal S, Siddique D. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061–2067. - PubMed
-
- Mallucci G, Collinge J. Rational targeting for prion therapeutics. Nat Rev Neurosci. 2005;6:23–34. - PubMed
-
- Trevitt C, Collinge J. A systematic review of prion therapeutics in experimental models. Brain. 2006;129:2241–2265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

