Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images
- PMID: 19278927
- PMCID: PMC2911837
- DOI: 10.1109/TMI.2009.2016958
Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images
Abstract
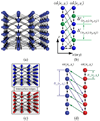
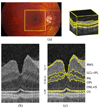
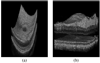
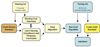
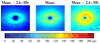
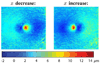
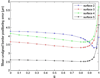
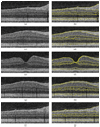
With the introduction of spectral-domain optical coherence tomography (OCT), much larger image datasets are routinely acquired compared to what was possible using the previous generation of time-domain OCT. Thus, the need for 3-D segmentation methods for processing such data is becoming increasingly important. We report a graph-theoretic segmentation method for the simultaneous segmentation of multiple 3-D surfaces that is guaranteed to be optimal with respect to the cost function and that is directly applicable to the segmentation of 3-D spectral OCT image data. We present two extensions to the general layered graph segmentation method: the ability to incorporate varying feasibility constraints and the ability to incorporate true regional information. Appropriate feasibility constraints and cost functions were learned from a training set of 13 spectral-domain OCT images from 13 subjects. After training, our approach was tested on a test set of 28 images from 14 subjects. An overall mean unsigned border positioning error of 5.69+/-2.41 microm was achieved when segmenting seven surfaces (six layers) and using the average of the manual tracings of two ophthalmologists as the reference standard. This result is very comparable to the measured interobserver variability of 5.71+/-1.98 microm.
Figures





References
-
- Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J. Biomed. Opt. 2002 Jul;vol. 7(no. 3):457–463. - PubMed
-
- de Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt. Lett. 2003 Nov;vol. 28(no. 21):2067–2069. - PubMed
-
- Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Progress Retinal Eye Res. 2008;vol. 27(no. 1):45–88. - PubMed
-
- Koozekanani D, Boyer K, Roberts C. Retinal thickness measurements in optical coherence tomography using a Markov boundary model. Proc. IEEE Conf. Comput. Vis. Pattern Recognit. 2000 Jun;vol. 2:363–370. - PubMed
-
- Koozekanani D, Boyer K, Roberts C. Retinal thickness measurements from optical coherence tomography using a Markov boundary model. IEEE Trans. Med. Imag. 2001 Sep;vol. 20(no. 9):900–916. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

