Diagnosis of Fanconi anemia in patients with bone marrow failure
- PMID: 19278965
- PMCID: PMC2663612
- DOI: 10.3324/haematol.13592
Diagnosis of Fanconi anemia in patients with bone marrow failure
Abstract
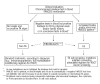
Background: Patients with bone marrow failure and undiagnosed underlying Fanconi anemia may experience major toxicity if given standard-dose conditioning regimens for hematopoietic stem cell transplant. Due to clinical variability and/or potential emergence of genetic reversion with hematopoietic somatic mosaicism, a straightforward Fanconi anemia diagnosis can be difficult to make, and diagnostic strategies combining different assays in addition to classical breakage tests in blood may be needed.
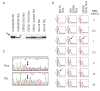
Design and methods: We evaluated Fanconi anemia diagnosis on blood lymphocytes and skin fibroblasts from a cohort of 87 bone marrow failure patients (55 children and 32 adults) with no obvious full clinical picture of Fanconi anemia, by performing a combination of chromosomal breakage tests, FANCD2-monoubiquitination assays, a new flow cytometry-based mitomycin C sensitivity test in fibroblasts, and, when Fanconi anemia was diagnosed, complementation group and mutation analyses. The mitomycin C sensitivity test in fibroblasts was validated on control Fanconi anemia and non-Fanconi anemia samples, including other chromosomal instability disorders.
Results: When this diagnosis strategy was applied to the cohort of bone marrow failure patients, 7 Fanconi anemia patients were found (3 children and 4 adults). Classical chromosomal breakage tests in blood detected 4, but analyses on fibroblasts were necessary to diagnose 3 more patients with hematopoietic somatic mosaicism. Importantly, Fanconi anemia was excluded in all the other patients who were fully evaluated.
Conclusions: In this large cohort of patients with bone marrow failure our results confirmed that when any clinical/biological suspicion of Fanconi anemia remains after chromosome breakage tests in blood, based on physical examination, history or inconclusive results, then further evaluation including fibroblast analysis should be made. For that purpose, the flow-based mitomycin C sensitivity test here described proved to be a reliable alternative method to evaluate Fanconi anemia phenotype in fibroblasts. This global strategy allowed early and accurate confirmation or rejection of Fanconi anemia diagnosis with immediate clinical impact for those who underwent hematopoietic stem cell transplant.
Figures


References
-
- Alter BP. Inherited bone marrow failure syndromes. In: Nathan DG, Ginsburg D, Orkin SH, Look AT, editors. Hematology of Infancy and Childhood. 6th ed. Philadelphia: Saunders; 2003. pp. 280–365.
-
- Dokal I, Vulliamy T. Inherited aplastic anaemias/bone marrow failure syndromes. Blood Rev. 2008;22:141–53. - PubMed
-
- Giampietro PF, Adler-Brecher B, Verlander PC, Pavlakis SG, Davis JG, Auerbach AD. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

