Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment?
- PMID: 19279047
- PMCID: PMC2725755
- DOI: 10.1093/humupd/dmp004
Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment?
Abstract
Background: Adaptation of the maternal immune response to accommodate the semi-allogeneic fetus is necessary for pregnancy success, and disturbances in maternal tolerance are implicated in infertility and reproductive pathologies. T regulatory (Treg) cells are a recently discovered subset of T-lymphocytes with potent suppressive activity and pivotal roles in curtailing destructive immune responses and preventing autoimmune disease.
Methods: A systematic review was undertaken of the published literature on Treg cells in the ovary, testes, uterus and gestational tissues in pregnancy, and their link with infertility, miscarriage and pathologies of pregnancy. An overview of current knowledge on the generation, activation and modes of action of Treg cells in controlling immune responses is provided, and strategies for manipulating regulatory T-cells for potential applications in reproductive medicine are discussed.
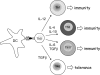
Results: Studies in mouse models show that Treg cells are essential for maternal tolerance of the conceptus, and that expansion of the Treg cell pool through antigen-specific and antigen non-specific pathways allows their suppressive actions to be exerted in the critical peri-implantation phase of pregnancy. In women, Treg cells accumulate in the decidua and are elevated in maternal blood from early in the first trimester. Inadequate numbers of Treg cells or their functional deficiency are linked with infertility, miscarriage and pre-eclampsia.
Conclusions: The potency and wide-ranging involvement of Treg cells in immune homeostasis and disease pathology indicates the considerable potential of these cells as therapeutic agents, raising the prospect of their utility in novel treatments for reproductive pathologies.
Figures



References
-
- Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–237. - PubMed
-
- Alard P, Thompson C, Agersborg SS, Thatte J, Setiady Y, Samy E, Tung KS. Endogenous oocyte antigens are required for rapid induction and progression of autoimmune ovarian disease following day-3 thymectomy. J Immunol. 2001;166:4363–4369. - PubMed
-
- Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

