CD4+ T cells are required for the priming of CD8+ T cells following infection with herpes simplex virus type 1
- PMID: 19279095
- PMCID: PMC2682109
- DOI: 10.1128/JVI.01997-08
CD4+ T cells are required for the priming of CD8+ T cells following infection with herpes simplex virus type 1
Abstract
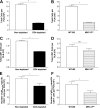
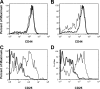
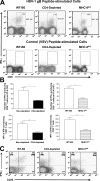
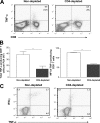
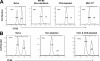
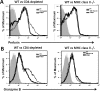
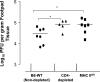
The role of CD4(+) helper T cells in modulating the acquired immune response to herpes simplex virus type 1 (HSV-1) remains ill defined; in particular, it is unclear whether CD4(+) T cells are needed for the generation of the protective HSV-1-specific CD8(+)-T-cell response. This study examined the contribution of CD4(+) T cells in the generation of the primary CD8(+)-T-cell responses following acute infection with HSV-1. The results demonstrate that the CD8(+)-T-cell response generated in the draining lymph nodes of CD4(+)-T-cell-depleted C57BL/6 mice and B6-MHC-II(-/-) mice is quantitatively and qualitatively distinct from the CD8(+) T cells generated in normal C57BL/6 mice. Phenotypic analyses show that virus-specific CD8(+) T cells express comparable levels of the activation marker CD44 in mice lacking CD4(+) T cells and normal mice. In contrast, CD8(+) T cells generated in the absence of CD4(+) T cells express the interleukin 2 receptor alpha-chain (CD25) at lower levels. Importantly, the CD8(+) T cells in the CD4(+)-T-cell-deficient environment are functionally active with respect to the expression of cytolytic activity in vivo but exhibit a diminished capacity to produce gamma interferon and tumor necrosis factor alpha. Furthermore, the primary expansion of HSV-1-specific CD8(+) T cells is diminished in the absence of CD4(+)-T-cell help. These results suggest that CD4(+)-T-cell help is essential for the generation of fully functional CD8(+) T cells during the primary response to HSV-1 infection.
Figures


Similar articles
-
Diminished secondary CTL response in draining lymph nodes on cutaneous challenge with herpes simplex virus.J Gen Virol. 2000 Feb;81(Pt 2):407-14. doi: 10.1099/0022-1317-81-2-407. J Gen Virol. 2000. PMID: 10644839
-
Either a CD4(+)or CD8(+)T cell function is sufficient for clearance of infectious virus from trigeminal ganglia and establishment of herpes simplex virus type 1 latency in mice.Microb Pathog. 1999 Dec;27(6):387-94. doi: 10.1006/mpat.1999.0314. Microb Pathog. 1999. PMID: 10588911
-
Herpes simplex virus (HSV)-specific T cells activated in the absence of IFN-gamma express alternative effector functions but are not protective against genital HSV-2 infection.J Reprod Immunol. 2010 Jan;84(1):8-15. doi: 10.1016/j.jri.2009.09.007. Epub 2009 Nov 25. J Reprod Immunol. 2010. PMID: 19942296 Free PMC article.
-
Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection.J Zhejiang Univ Sci B. 2017 Apr.;18(4):277-288. doi: 10.1631/jzus.B1600460. J Zhejiang Univ Sci B. 2017. PMID: 28378566 Free PMC article. Review.
-
Immunology in the Clinic Review Series; focus on host responses: T cell responses to herpes simplex viruses.Clin Exp Immunol. 2012 Jan;167(1):47-58. doi: 10.1111/j.1365-2249.2011.04502.x. Clin Exp Immunol. 2012. PMID: 22132884 Free PMC article. Review.
Cited by
-
Critical role of microRNA-155 in herpes simplex encephalitis.J Immunol. 2014 Mar 15;192(6):2734-43. doi: 10.4049/jimmunol.1302326. Epub 2014 Feb 10. J Immunol. 2014. PMID: 24516198 Free PMC article.
-
A Functional Type I Interferon Pathway Drives Resistance to Cornea Herpes Simplex Virus Type 1 Infection by Recruitment of Leukocytes.J Biomed Res. 2011 Mar;25(2):111-119. doi: 10.1016/s1674-8301(11)60014-6. J Biomed Res. 2011. PMID: 21709805 Free PMC article.
-
Upregulation of Multiple CD8+ T Cell Exhaustion Pathways Is Associated with Recurrent Ocular Herpes Simplex Virus Type 1 Infection.J Immunol. 2020 Jul 15;205(2):454-468. doi: 10.4049/jimmunol.2000131. Epub 2020 Jun 15. J Immunol. 2020. PMID: 32540992 Free PMC article.
-
CD8+ T cells suppress viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis.J Immunol. 2012 Jul 1;189(1):425-32. doi: 10.4049/jimmunol.1200063. Epub 2012 May 30. J Immunol. 2012. PMID: 22649204 Free PMC article.
-
Human Epitopes Identified from Herpes Simplex Virus Tegument Protein VP11/12 (UL46) Recall Multifunctional Effector Memory CD4+ TEM Cells in Asymptomatic Individuals and Protect from Ocular Herpes Infection and Disease in "Humanized" HLA-DR Transgenic Mice.J Virol. 2020 Mar 17;94(7):e01991-19. doi: 10.1128/JVI.01991-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915285 Free PMC article.
References
-
- Andersen, H., D. Dempsey, R. Chervenak, and S. R. Jennings. 2000. Expression of intracellular IFN-gamma in HSV-1-specific CD8+ T cells identifies distinct responding subpopulations during the primary response to infection. J. Immunol. 1652101-2107. - PubMed
-
- Belz, G. T., H. Liu, S. Andreansky, P. C. Doherty, and P. G. Stevenson. 2003. Absence of a functional defect in CD8+ T cells during primary murine gammaherpesvirus-68 infection of I-A (b−/−) mice. J. Gen. Virol. 84337-341. - PubMed
-
- Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393478-480. - PubMed
-
- Bevan, M. J. 2004. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 4595-602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

