Impact of human immunodeficiency virus type 1 resistance to protease inhibitors on evolution of resistance to the maturation inhibitor bevirimat (PA-457)
- PMID: 19279107
- PMCID: PMC2682084
- DOI: 10.1128/JVI.02659-08
Impact of human immunodeficiency virus type 1 resistance to protease inhibitors on evolution of resistance to the maturation inhibitor bevirimat (PA-457)
Abstract
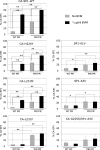
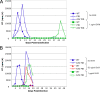
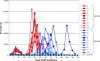
The maturation inhibitor bevirimat [3-O-(3',3'dimethysuccinyl)betulinic acid; BVM; also known as PA-457 or DSB] potently inhibits human immunodeficiency virus type 1 (HIV-1) replication by blocking protease (PR)-mediated cleavage at the junction between capsid (CA) and spacer peptide 1 (SP1) in Gag. We previously isolated a panel of single-amino-acid substitutions that confer resistance to BVM in vitro (C. S. Adamson, S. D. Ablan, I. Boeras, R. Goila-Gaur, F. Soheilian, K. Nagashima, F. Li, K. Salzwedel, M. Sakalian, C. T. Wild, and E. O. Freed, J. Virol. 80:10957-10971, 2006). The BVM resistance mutations cluster at or near the CA-SP1 cleavage site. Because BVM likely will be used clinically in patients harboring viruses resistant to PR inhibitors (PIs), in this study we evaluated the interplay between a PI-resistant (PIR) PR and the BVM resistance mutations in Gag. As expected, the PIR mutations had no effect on inhibition by BVM; however, we observed general processing defects and a slight delay in viral replication in Jurkat T cells associated with the PIR mutations, even in the absence of compound. When combined, most BVM resistance and PIR mutations acted additively to impair viral replication, particularly in the presence of BVM. The BVM-resistant mutant SP1-A1V was an exception, as it supported robust replication in the context of either wild-type (WT) or PIR PR, even at high BVM concentrations. Significantly, the emergence of BVM resistance was delayed in the context of the PIR PR, and the SP1-A1V mutation was acquired most frequently with either WT or PIR PR. These results suggest that resistance to BVM is less likely to emerge in patients who have failed PIs than in patients who are PI naive. We predict that the SP1-A1V substitution is the most likely to emerge in vivo, as this mutant replicates robustly independently of PR mutations or BVM. These findings offer insights into the effect of PIR mutations on the evolution of BVM resistance in PI-experienced patients.
Figures



References
-
- Adamson, C. S., and E. O. Freed. 2007. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv. Pharmacol. 55347-387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

