An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells
- PMID: 19279219
- PMCID: PMC2654393
- DOI: 10.1073/pnas.0810614106
An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells
Abstract
Production of clinical-grade gene therapy vectors for human trials remains a major hurdle in advancing cures for a number of otherwise incurable diseases. We describe a system based on a stably transformed insect cell lines harboring helper genes required for vector production. Integrated genes remain silent until the cell is infected with a single baculovirus expression vector (BEV). The induction of expression results from a combination of the amplification of integrated resident genes (up to 1,200 copies per cell) and the enhancement of the expression mediated by the immediate-early trans-regulator 1 (IE-1) encoded by BEV. The integration cassette incorporates an IE-1 binding target sequence from wild-type Autographa californica multiple nuclear polyhedrosis virus, a homologous region 2 (hr2). A feed-forward loop is initiated by one of the induced proteins, Rep78, boosting the amplification of the integrated genes. The system was tested for the coordinated expression of 7 proteins required to package recombinant adeno-associated virus (rAAV)2 and rAAV1. The described arrangement provided high levels of Rep and Cap proteins, thus improving rAAV yield by 10-fold as compared with the previously described baculovirus/rAAV production system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
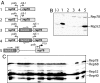
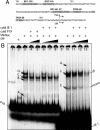


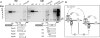
References
-
- Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol. 2004;22:1583–1587. - PubMed
-
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. - PubMed
-
- Cecchini S, Negrete A, Kotin RM. Toward exascale production of recombinant adeno-associated virus for gene transfer applications. Gene Ther. 2008;15:823–830. - PubMed
-
- Chen H. Intron splicing-mediated expression of AAV Rep and Cap genes and production of AAV vectors in insect cells. Mol Ther. 2008;16:924–930. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

