Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3K
- PMID: 19279233
- PMCID: PMC2681376
- DOI: 10.1152/ajpcell.00291.2008
Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3K
Abstract
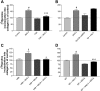
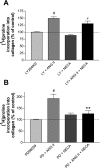
Rat cardiac fibroblasts (CF) express multiple adenosine (ADO) receptors. Pharmacological evidence suggests that activation of A(2) receptors may inhibit collagen synthesis via adenylyl cyclase-induced elevation of cellular cAMP. We have characterized the signaling pathways involved in ADO-mediated inhibition of collagen synthesis in primary cultures of adult rat CF. ANG II stimulates collagen production in these cells. Coincubation with agents that elevate cellular cAMP [the ADO agonist, 5'-N-ethylcarboxamidoadensoine (NECA), and forskolin] inhibited the stimulatory effects of ANG II. However, direct stimulators and inhibitors of protein kinase A (PKA) did not alter ANG II-induced collagen synthesis, indicating that PKA does not mediate the inhibitory effects of NECA. Inhibitors of AMP-kinase (AMPK) and extracellular signal-regulated kinase 1/2 (ERK1/2) do not alter NECA-inhibited collagen synthesis. However, activation of exchange factor directly activated by cAMP (Epac) mimicked the effects of NECA on ANG II-stimulated collagen synthesis. Inhibition of phosphoinositol-3 kinase (PI3K) reduced the inhibitory effects of NECA on ANG II-induced collagen synthesis, suggesting that NECA acts via PI3K. Furthermore, inhibition of PI3K also relieved the inhibitory effect of Epac activation on ANG II-stimulated collagen synthesis. Thus it appears that ADO activates the A(2)R-G(s)-adenylyl cyclase pathway and that the resultant cAMP reduces collagen synthesis via a PKA-independent, Epac-dependent pathway that feeds through PI3K.
Figures





References
-
- Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol 15: 343–349, 2005. - PubMed
-
- Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J 16, Suppl O: 107–109, 1995. - PubMed
-
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45: 657–687, 2005. - PubMed
-
- Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem 186: 129–136, 1989. - PubMed
-
- Carroll EP, Janicki JS, Pick R, Weber KT. Myocardial stiffness and reparative fibrosis following coronary embolisation in the rat. Cardiovasc Res 23: 655–661, 1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

