GABA-induced intersubunit conformational movement in the GABAA receptor alpha 1M1-beta 2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anesthetic binding site
- PMID: 19279245
- PMCID: PMC2677711
- DOI: 10.1523/JNEUROSCI.6090-08.2009
GABA-induced intersubunit conformational movement in the GABAA receptor alpha 1M1-beta 2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anesthetic binding site
Abstract
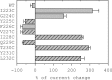
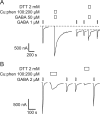
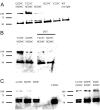
The molecular basis of general anesthetic interactions with GABA(A) receptors is uncertain. An accurate homology model would facilitate studies of anesthetic action. Construction of a GABA(A) model based on the 4 A resolution acetylcholine receptor structure is complicated by alignment uncertainty between the acetylcholine and GABA(A) receptor M3 and M4 transmembrane segments. Using disulfide crosslinking we previously established the orientation of M2 and M3 within a single GABA(A) subunit. The resultant model predicts that the betaM3 residue beta2M286, implicated in anesthetic binding, faces the adjacent alpha1-M1 segment and not into the beta2 subunit interior as some models have suggested. To assess the proximity of beta2M286 to the alpha1-M1 segment we expressed beta2M286C and gamma2 with 10 consecutive alpha1-M1 cysteine (Cys) mutants, alpha1I223C to alpha1L232C, in and flanking the extracellular end of alpha1-M1. In activated states, beta2M286C formed disulfide bonds with alpha1Y225C and alpha1Q229C based on electrophysiological assays and dimers on Western blots, but not with other alpha1-M1 mutants. beta2F289, one helical turn below beta2M286, formed disulfide bonds with alpha1I228C, alpha1Q229C and alpha1L232C in activated states. The intervening residues, beta2G287C and beta2C288, did not form disulfide bonds with alpha1-M1 Cys mutants. We conclude that the beta2-M3 residues beta2M286 and beta2F289 face the intersubunit interface in close proximity to alpha1-M1 and that channel gating induces a structural rearrangement in the transmembrane subunit interface that reduces the betaM3 to alphaM1 separation by approximately 7 A. This supports the hypothesis that some intravenous anesthetics bind in the betaM3-alphaM1 subunit interface consistent with azi-etomidate photoaffinity labeling.
Figures





References
-
- Akabas MH. GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. - PubMed
-
- Akabas MH, Karlin A. Identification of acetylcholine receptor channel-lining residues in the M1 segment of the alpha-subunit. Biochemistry. 1995;34:12496–12500. - PubMed
-
- Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
