Dynamic encoding of action selection by the medial striatum
- PMID: 19279252
- PMCID: PMC3415331
- DOI: 10.1523/JNEUROSCI.5206-08.2009
Dynamic encoding of action selection by the medial striatum
Abstract
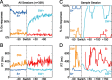
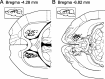
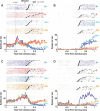
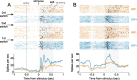
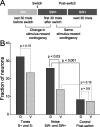
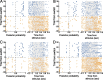
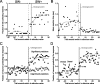
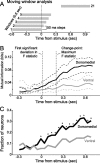
Successful foragers respond flexibly to environmental stimuli. Behavioral flexibility depends on a number of brain areas that send convergent projections to the medial striatum, such as the medial prefrontal cortex, orbital frontal cortex, and amygdala. Here, we tested the hypothesis that neurons in the medial striatum are involved in flexible action selection, by representing changes in stimulus-reward contingencies. Using a novel Go/No-go reaction-time task, we changed the reward value of individual stimuli within single experimental sessions. We simultaneously recorded neuronal activity in the medial and ventral parts of the striatum of rats. The rats modified their actions in the task after the changes in stimulus-reward contingencies. This was preceded by dynamic modulations of spike activity in the medial, but not the ventral, striatum. Our results suggest that the medial striatum biases animals to collect rewards to potentially valuable stimuli and can rapidly influence flexible behavior.
Figures

Similar articles
-
Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review.
-
Multifaceted Contributions by Different Regions of the Orbitofrontal and Medial Prefrontal Cortex to Probabilistic Reversal Learning.J Neurosci. 2016 Feb 10;36(6):1996-2006. doi: 10.1523/JNEUROSCI.3366-15.2016. J Neurosci. 2016. PMID: 26865622 Free PMC article.
-
Inactivation of the lateral but not medial dorsal striatum eliminates the excitatory impact of Pavlovian stimuli on instrumental responding.J Neurosci. 2007 Dec 19;27(51):13977-81. doi: 10.1523/JNEUROSCI.4097-07.2007. J Neurosci. 2007. PMID: 18094235 Free PMC article.
-
Ventral striatum encodes past and predicted value independent of motor contingencies.J Neurosci. 2012 Feb 8;32(6):2027-36. doi: 10.1523/JNEUROSCI.5349-11.2012. J Neurosci. 2012. PMID: 22323717 Free PMC article.
-
Contributions of the striatum to learning, motivation, and performance: an associative account.Trends Cogn Sci. 2012 Sep;16(9):467-75. doi: 10.1016/j.tics.2012.07.007. Epub 2012 Aug 10. Trends Cogn Sci. 2012. PMID: 22890090 Free PMC article. Review.
Cited by
-
Reinforcement learning approaches to hippocampus-dependent flexible spatial navigation.Brain Neurosci Adv. 2021 Apr 9;5:2398212820975634. doi: 10.1177/2398212820975634. eCollection 2021 Jan-Dec. Brain Neurosci Adv. 2021. PMID: 33954259 Free PMC article.
-
Distinct recruitment of dorsomedial and dorsolateral striatum erodes with extended training.Elife. 2019 Oct 17;8:e49536. doi: 10.7554/eLife.49536. Elife. 2019. PMID: 31621583 Free PMC article.
-
Novelty encoding by the output neurons of the Basal Ganglia.Front Syst Neurosci. 2010 Jan 8;3:20. doi: 10.3389/neuro.06.020.2009. eCollection 2010. Front Syst Neurosci. 2010. PMID: 20140267 Free PMC article.
-
Integrating early results on ventral striatal gamma oscillations in the rat.Front Neurosci. 2010 Sep 15;4:300. doi: 10.3389/fnins.2010.00300. eCollection 2010. Front Neurosci. 2010. PMID: 21350600 Free PMC article.
-
Neuronal correlates of instrumental learning in the dorsal striatum.J Neurophysiol. 2009 Jul;102(1):475-89. doi: 10.1152/jn.00262.2009. Epub 2009 May 13. J Neurophysiol. 2009. PMID: 19439679 Free PMC article.
References
-
- Adams S, Kesner RP, Ragozzino ME. Role of the medial and lateral caudate-putamen in mediating an auditory conditional response association. Neurobiol Learn Mem. 2001;76:106–116. - PubMed
-
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. - PubMed
-
- Barry D, Hartigan JA. A Bayesian analysis for change point problems. J Am Stat Assoc. 1993;88:309–319.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
