Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy
- PMID: 19279253
- PMCID: PMC2683764
- DOI: 10.1523/JNEUROSCI.3941-08.2009
Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy
Abstract
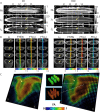
Diffusion tensor imaging (DTI) and immunohistochemistry were used to examine axon injury in the rat spinal cord after unilateral L(2)-L(4) dorsal root axotomy at multiple time points (from 16 h to 30 d after surgery). Three days after axotomy, DTI revealed a lesion in the ipsilateral dorsal column extending from the lumbar to the cervical cord. The lesion showed significantly reduced parallel diffusivity and increased perpendicular diffusivity at day 3 compared with the contralateral unlesioned dorsal column. These findings coincided with loss of phosphorylated neurofilaments, accumulation of nonphosphorylated neurofilaments, swollen axons and formation of myelin ovoids, and no clear loss of myelin (stained by Luxol fast blue and 2'-3'-cyclic nucleotide 3'-phosphodiesterase). At day 30, DTI of the lesion continued to show significantly decreased parallel diffusivity. There was a slow but significant increase in perpendicular diffusivity between day 3 and day 30, which correlated with gradual clearance of myelin without further significant changes in neurofilament levels. These results show that parallel diffusivity can detect axon degeneration within 3 d after injury. The clearance of myelin at later stages may contribute to the late increase in perpendicular diffusivity, whereas the cause of its early increase at day 3 may be related to changes associated with primary axon injury. These data suggest that there is an early imaging signature associated with axon transections that could be used in a variety of neurological disease processes.
Figures






References
-
- Agosta F, Absinta M, Sormani MP, Ghezzi A, Bertolotto A, Montanari E, Comi G, Filippi M. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007;130:2211–2219. - PubMed
-
- Asanuma T, Doblas S, Tesiram YA, Saunders D, Cranford R, Yasui H, Inanami O, Smith N, Floyd RA, Kotake Y, Towner RA. Visualization of the protective ability of a free radical trapping compound against rat C6 and F98 gliomas with diffusion tensor fiber tractography. J Magn Reson Imaging. 2008;28:574–587. - PubMed
-
- Assaf Y, Cohen Y. Non-mono-exponential attenuation of water and N-acetyl aspartate signals due to diffusion in brain tissue. J Magn Reson. 1998;131:69–85. - PubMed
-
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. - PubMed
-
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
