Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons
- PMID: 19279258
- PMCID: PMC2664544
- DOI: 10.1523/JNEUROSCI.4514-08.2009
Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons
Abstract
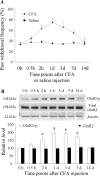
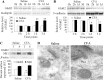
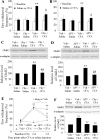
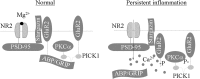
Spinal cord GluR2-lacking AMPA receptors (AMPARs) contribute to nociceptive hypersensitivity in persistent pain, but the molecular mechanisms underlying this event are not completely understood. We report that complete Freund's adjuvant (CFA)-induced peripheral inflammation induces synaptic GluR2 internalization in dorsal horn neurons during the maintenance of CFA-evoked nociceptive hypersensitivity. This internalization is initiated by GluR2 phosphorylation at Ser(880) and subsequent disruption of GluR2 binding to its synaptic anchoring protein (GRIP), resulting in a switch of GluR2-containing AMPARs to GluR2-lacking AMPARs and an increase of AMPAR Ca(2+) permeability at the synapses in dorsal horn neurons. Spinal cord NMDA receptor-mediated triggering of protein kinase C (PKC) activation is required for the induction and maintenance of CFA-induced dorsal horn GluR2 internalization. Moreover, preventing CFA-induced spinal GluR2 internalization through targeted mutation of the GluR2 PKC phosphorylation site impairs CFA-evoked nociceptive hypersensitivity during the maintenance period. These results suggest that dorsal horn GluR2 internalization might participate in the maintenance of NMDA receptor/PKC-dependent nociceptive hypersensitivity in persistent inflammatory pain.
Figures






References
-
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. - PubMed
-
- Basbaum AI, Woolf CJ. Pain. Curr Biol. 1999;9:R429–R431. - PubMed
-
- Beloeil H, Ji RR, Berde CB. Effects of bupivacaine and tetrodotoxin on carrageenan-induced hind paw inflammation in rats (Part 2): cytokines and p38 mitogen-activated protein kinases in dorsal root ganglia and spinal cord. Anesthesiology. 2006;105:139–145. - PubMed
-
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. - PubMed
-
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
