The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels
- PMID: 19279261
- PMCID: PMC3758885
- DOI: 10.1523/JNEUROSCI.4767-08.2009
The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels
Abstract
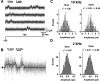
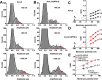
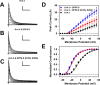
The neuronal subthreshold-operating A-type K(+) current regulates electrical excitability, spike timing, and synaptic integration and plasticity. The Kv4 channels underlying this current have been implicated in epilepsy, regulation of dopamine release, and pain plasticity. However, the unitary conductance (gamma) of neuronal somatodendritic A-type K(+) channels composed of Kv4 pore-forming subunits is larger (approximately 7.5 pS) than that of Kv4 channels expressed singly in heterologous cells (approximately 4 pS). Here, we examined the putative novel contribution of the dipeptidyl-peptidase-like protein-6 DPP6-S to the gamma of native [cerebellar granule neuron (CGN)] and reconstituted Kv4.2 channels. Coexpression of Kv4.2 proteins with DPP6-S was sufficient to match the gamma of native CGN channels; and CGN Kv4 channels from dpp6 knock-out mice yielded a gamma indistinguishable from that of Kv4.2 channels expressed singly. Moreover, suggesting electrostatic interactions, charge neutralization mutations of two N-terminal acidic residues in DPP6-S eliminated the increase in gamma. Therefore, DPP6-S, as a membrane protein extrinsic to the pore domain, is necessary and sufficient to explain a fundamental difference between native and recombinant Kv4 channels. These observations may help to understand the molecular basis of neurological disorders correlated with recently identified human mutations in the dpp6 gene.
Figures



References
-
- Abbott GW, Goldstein SA, Sesti F. Do all voltage-gated potassium channels use MiRPs? Circ Res. 2001;88:981–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
