Uniquely hominid features of adult human astrocytes
- PMID: 19279265
- PMCID: PMC2819812
- DOI: 10.1523/JNEUROSCI.4707-08.2009
Uniquely hominid features of adult human astrocytes
Abstract
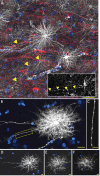
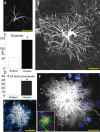
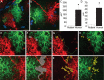
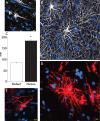
Defining the microanatomic differences between the human brain and that of other mammals is key to understanding its unique computational power. Although much effort has been devoted to comparative studies of neurons, astrocytes have received far less attention. We report here that protoplasmic astrocytes in human neocortex are 2.6-fold larger in diameter and extend 10-fold more GFAP (glial fibrillary acidic protein)-positive primary processes than their rodent counterparts. In cortical slices prepared from acutely resected surgical tissue, protoplasmic astrocytes propagate Ca(2+) waves with a speed of 36 microm/s, approximately fourfold faster than rodent. Human astrocytes also transiently increase cystosolic Ca(2+) in response to glutamatergic and purinergic receptor agonists. The human neocortex also harbors several anatomically defined subclasses of astrocytes not represented in rodents. These include a population of astrocytes that reside in layers 5-6 and extend long fibers characterized by regularly spaced varicosities. Another specialized type of astrocyte, the interlaminar astrocyte, abundantly populates the superficial cortical layers and extends long processes without varicosities to cortical layers 3 and 4. Human fibrous astrocytes resemble their rodent counterpart but are larger in diameter. Thus, human cortical astrocytes are both larger, and structurally both more complex and more diverse, than those of rodents. On this basis, we posit that this astrocytic complexity has permitted the increased functional competence of the adult human brain.
Figures





References
-
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. - PubMed
-
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
