Primary open-angle glaucoma
- PMID: 19279343
- PMCID: PMC3700399
- DOI: 10.1056/NEJMra0804630
Primary open-angle glaucoma
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

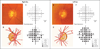

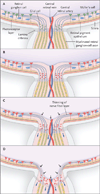
Comment in
-
Primary open-angle glaucoma.N Engl J Med. 2009 Jun 18;360(25):2679; author reply 2679-80. doi: 10.1056/NEJMc090757. N Engl J Med. 2009. PMID: 19535810 No abstract available.
-
Primary open-angle glaucoma.N Engl J Med. 2009 Jun 18;360(25):2679; author reply 2679-80. N Engl J Med. 2009. PMID: 19537321 No abstract available.
References
-
- Hayreh SS, Jonas JB. Optic disc morphology after arteritic anterior ischemic optic neuropathy. Ophthalmology. 2001;108:1586–1594. - PubMed
-
- Alward WL. Biomedicine: a new angle on ocular development. Science. 2003;299:1527–1528. - PubMed
-
- Fingert JH, Héon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. - PubMed
-
- Aldred MA, Baumber L, Hill A, et al. Low prevalence of MYOC mutations in UK primary open-angle glaucoma patients limits the utility of genetic testing. Hum Genet. 2004;115:428–431. - PubMed
-
- Graul TA, Kwon YH, Zimmerman MB, et al. A case-control comparison of the clinical characteristics of glaucoma and ocular hypertensive patients with and without the myocilin Gln368Stop mutation. Am J Ophthalmol. 2002;134:884–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
