CRF enhancement of GIRK channel-mediated transmission in dopamine neurons
- PMID: 19279570
- PMCID: PMC3640552
- DOI: 10.1038/npp.2009.25
CRF enhancement of GIRK channel-mediated transmission in dopamine neurons
Abstract
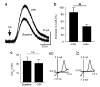
Dopamine neurons in the ventral midbrain contribute to learning and memory of natural and drug-related rewards. Corticotropin-releasing factor (CRF), a stress-related peptide, is thought to be involved in aspects of relapse following drug withdrawal, but the cellular actions are poorly understood. This study investigates the action of CRF on G-protein-linked inhibitory postsynaptic currents (IPSCs) mediated by GIRK (Kir3) channels in dopamine neurons. CRF enhanced the amplitude and slowed the kinetics of IPSCs following activation of D2-dopamine and GABA(B) receptors. This action was postsynaptic and dependent on the CRF(1) receptor. The enhancement induced by CRF was attenuated by repeated in vivo exposures to psychostimulants or restraint stress. The results indicate that CRF influences dopamine- and GABA-mediated inhibition in the midbrain, suggesting implications for the chronic actions of psychostimulants and stress on dopamine-mediated behaviors.
Figures

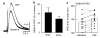
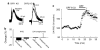
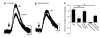
References
-
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehavioral Rev. 2004;28:415–431. - PubMed
-
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the ‘anxiogenic-like’ effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. - PubMed
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

