Coordination of dual incision and repair synthesis in human nucleotide excision repair
- PMID: 19279666
- PMCID: PMC2683701
- DOI: 10.1038/emboj.2009.49
Coordination of dual incision and repair synthesis in human nucleotide excision repair
Abstract
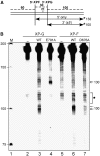
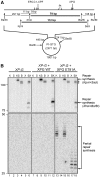
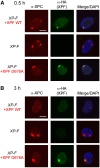
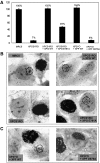
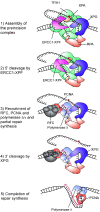
Nucleotide excision repair (NER) requires the coordinated sequential assembly and actions of the involved proteins at sites of DNA damage. Following damage recognition, dual incision 5' to the lesion by ERCC1-XPF and 3' to the lesion by XPG leads to the removal of a lesion-containing oligonucleotide of about 30 nucleotides. The resulting single-stranded DNA (ssDNA) gap on the undamaged strand is filled in by DNA repair synthesis. Here, we have asked how dual incision and repair synthesis are coordinated in human cells to avoid the exposure of potentially harmful ssDNA intermediates. Using catalytically inactive mutants of ERCC1-XPF and XPG, we show that the 5' incision by ERCC1-XPF precedes the 3' incision by XPG and that the initiation of repair synthesis does not require the catalytic activity of XPG. We propose that a defined order of dual incision and repair synthesis exists in human cells in the form of a 'cut-patch-cut-patch' mechanism. This mechanism may aid the smooth progression through the NER pathway and contribute to genome integrity.
Figures


References
-
- Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hübscher U, Egly JM, Wood RD (1995) Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell 80: 859–868 - PubMed
-
- Bardwell AJ, Bardwell L, Tomkinson AE, Friedberg EC (1994) Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science 265: 2082–2085 - PubMed
-
- Biggerstaff M, Wood RD (1999) Assay for nucleotide excision repair protein activity using fractionated cell extracts and UV-damaged plasmid DNA. Methods Mol Biol 113: 357–372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

