Organogenesis of kidney and endocrine pancreas: the window opens
- PMID: 19279701
- PMCID: PMC2649619
- DOI: 10.4161/org.3.2.5382
Organogenesis of kidney and endocrine pancreas: the window opens
Abstract
Growing new organs in situ by implanting developing animal organ primordia (organogenesis) represents a novel solution to the problem of limited supply for human donor organs that offers advantages relative to transplanting embryonic stem (ES) cells or xenotransplantation of developed organs. Successful transplantation of organ primordia depends on obtaining them at defined windows during embryonic development within which the risk of teratogenicity is eliminated, growth potential is maximized, and immunogenicity is reduced. We and others have shown that renal primordia transplanted into the mesentery undergo differentiation and growth, become vascularized by blood vessels of host origin, exhibit excretory function and support life in otherwise anephric hosts. Renal primordia can be transplanted across isogeneic, allogeneic or xenogeneic barriers. Pancreatic primordia can be transplanted across the same barriers undergo growth, and differentiation of endocrine components only and secrete insulin in a physiological manner following mesenteric placement. Insulin-secreting cells originating from embryonic day (E) 28 (E28) pig pancreatic primordia transplanted into the mesentery of streptozotocin-diabetic (type 1) Lewis rats or ZDF diabetic (type 2) rats or STZ-diabetic rhesus macaques engraft without the need for host immune-suppression. Our findings in diabetic macaques represent the first steps in the opening of a window for a novel treatment of diabetes in humans.
Keywords: chronic kidney disease; diabetes mellitus; non-human primates; transplantation; xenotransplantation; β-cell.
Figures

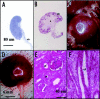
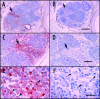
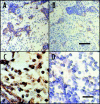
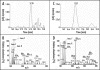

References
-
- Hammerman MR. Windows of opportunity for organogenesis. Transplant Immunology. 2005;5:1–8. - PubMed
-
- Rogers SA, Chen F, Talcott MR, Faulkner C, Thomas JM, Thevis M, Hammerman MR. Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques. Xenotransplantation. 2007;14:591–602. - PubMed
-
- Hammerman MR. Treatment for end-stage renal disease: An organogenesis/tissue engineering odyssey. Transpl Immunol. 2004;12:211–218. - PubMed
-
- Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DKC. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–499. - PubMed
-
- Cozzi E, Bhatti F, Schmoekel M, Chavez G, Smith KG, Zaidi A, Bradley JR, Thiru S, Goddard M, Vial C, Ostlie D, Wallwork J, White D, Friend PJ. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70:15–21. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
