Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE(-/-) mouse: implications for a "multihit" hypothesis of fatty liver disease
- PMID: 19280709
- PMCID: PMC2775483
- DOI: 10.1016/j.freeradbiomed.2009.01.003
Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE(-/-) mouse: implications for a "multihit" hypothesis of fatty liver disease
Abstract
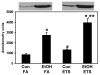
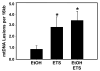
Although epidemiologic studies indicate that combined exposure to cigarette smoke and alcohol increase the risk and severity of liver diseases, the molecular mechanisms responsible for hepatotoxicity are unknown. Similarly, emerging evidence indicates a linkage among hepatic steatosis and cardiovascular disease. Herein, we hypothesize that combined exposure to alcohol and environmental tobacco smoke (ETS) on a hypercholesterolemic background increases liver injury through oxidative/nitrative stress, hypoxia, and mitochondrial damage. To test this, male apoE(-/-) mice were exposed to an ethanol-containing diet, ETS alone, or a combination of the two, and histology and functional endpoints were compared to filtered-air-exposed, ethanol-naïve controls.Whereas ethanol consumption induced a mild steatosis, combined exposure to ethanol + ETS resulted in increased hepatic steatosis, inflammation, alpha-smooth muscle actin, and collagen. Exposure to ethanol + ETS induced the largest increase in CYP2E1 and iNOS protein, as well as increased 3-nitrotyrosine, mtDNA damage, and decreased cytochrome c oxidase protein, compared to all other groups. Similarly, the largest increase in HIF1alpha expression was observed in the ethanol + ETS group, indicating enhanced hypoxia. These studies demonstrate that ETS increases alcohol-dependent steatosis and hypoxic stress. Therefore, ETS may be a key environmental "hit" that accelerates and exacerbates alcoholic liver disease in hypercholesterolemic apoE(-/-) mice.
Figures

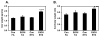






References
-
- Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22 1:S53–56. - PubMed
-
- Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310–314. - PubMed
-
- Bailey SM. A review of the role of reactive oxygen and nitrogen species in alcohol-induced mitochondrial dysfunction. Free Radic Res. 2003;37:585–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

