Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease
- PMID: 19281080
- PMCID: PMC2650609
- DOI: 10.2147/copd.s4480
Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease
Abstract
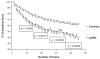
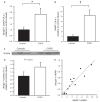
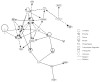
Chronic obstructive pulmonary disease (COPD) is a debilitating disease characterized by inflammation-induced airflow limitation and parenchymal destruction. In addition to pulmonary manifestations, patients with COPD develop systemic problems, including skeletal muscle and other organ-specific dysfunctions, nutritional abnormalities, weight loss, and adverse psychological responses. Patients with COPD often complain of dyspnea on exertion, reduced exercise capacity, and develop a progressive decline in lung function with increasing age. These symptoms have been attributed to increases in the work of breathing and in impairments in gas exchange that result from airflow limitation and dynamic hyperinflation. However, there is mounting evidence to suggest that skeletal muscle dysfunction, independent of lung function, contributes significantly to reduced exercise capacity and poor quality of life in these patients. Limb and ventilatory skeletal muscle dysfunction in COPD patients has been attributed to a myriad of factors, including the presence of low grade systemic inflammatory processes, nutritional depletion, corticosteroid medications, chronic inactivity, age, hypoxemia, smoking, oxidative and nitrosative stresses, protein degradation and changes in vascular density. This review briefly summarizes the contribution of these factors to overall skeletal muscle dysfunction in patients with COPD, with particular attention paid to the latest advances in the field.
Figures



References
-
- Agusti AG, Sauleda J, Miralles C, et al. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:485–9. - PubMed
-
- Alexopoulou C, Mitrouska I, Arvanitis D, et al. Vascular-Specific growth factor mRNA levels in the human diaphragm. Respiration. 2005;72:636–41. - PubMed
-
- Allaire J, Maltais F, LeBlanc P, et al. Lipofuscin accumulation in the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Muscle Nerve. 2002;25:383–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

