Tiotropium and exercise training in COPD patients: effects on dyspnea and exercise tolerance
- PMID: 19281092
- PMCID: PMC2650608
- DOI: 10.2147/copd.s3935
Tiotropium and exercise training in COPD patients: effects on dyspnea and exercise tolerance
Abstract
Background: Exercise training improves exercise tolerance in chronic obstructive pulmonary disease (COPD). Tiotropium 18 microg once daily induces sustained bronchodilation throughout the day and reduces hyperinflation, one of the pathophysiological factors contributing to exertional dyspnea in COPD patients.
Aim: To determine whether tiotropium enhances the effects of exercise training in patients with COPD.
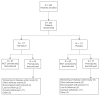
Design: Multicenter, 25 week randomized, double-blind, placebo-controlled, parallel-group study.
Setting: Twelve Italian Pulmonary Units practicing pulmonary rehabilitation.
Patients and intervention: Two hundred thirty four COPD patients (196 males; mean age: 67.4 +/- 7.6; forced expiratory volume at 1 second (FEV1): 41.4 +/- 13.0% predicted) were randomised to tiotropium 18 microg or placebo inhalation capsules taken once daily. Both groups underwent a 8 week pulmonary rehabilitation program (PR) consisting of 3 exercise training session per week.
Measurements: Baseline, at the end of PR and after 12 weeks, patients completed pulmonary function testing, six minute walking test (6MWT), the Baseline and Transition Dyspnea Index (BDI and TDI), and the St. George's Respiratory Questionnaire (SGRQ).
Results: Relative to placebo, tiotropium had larger trough and post-study drug FEV1 responses on all test days. At the end of and 12 weeks following PR, patients on tiotropium showed no statistically significant differences in 6MWT compared to patients on placebo. Compared to the period immediately prior to PR, the mean improvement in 6MWT was only 29.7 meters (7.1%) for the combined cohort. Mean TDI focal scores at the end of PR were 3.60 for tiotropium and 2.25 for placebo (p < 0.01). At 12 weeks after PR, TDI focal scores were 2.71 for tiotropium and 2.11 for placebo (p = 0.16). Reduction in all four SGRQ component scores, indicating an improvement in health-related quality of life, was observed for the tiotropium group over the duration of the study compared to placebo but the differences were not statistically significant. During the study period, there were fewer exacerbations and exacerbation days in the tiotropium group.
Conclusion: Although significant improvements were observed with perceived dyspnea, compared to placebo, the addition of tiotropium to pulmonary rehabilitation did not improve the 6MWT.
Figures




References
-
- [ATS] American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–36. - PubMed
-
- [ATS] American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1995;152:S77–S120. - PubMed
-
- [ATS] American Thoracic Society. ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–7. - PubMed
-
- Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone–salmeterol for treatment of Chronic Obstructive Pulmonary Disease. A randomized trial. Ann Intern Med. 2007;146:545–55. - PubMed
-
- Ambrosino N, Serradori M. Dyspnea, linguistic and biological descriptors. Chron Respir Dis. 2006;3:117–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

