Method revealing bacterial cell-wall architecture by time-dependent isotope labeling and quantitative liquid chromatography/mass spectrometry
- PMID: 19281243
- PMCID: PMC2715431
- DOI: 10.1021/ac802587r
Method revealing bacterial cell-wall architecture by time-dependent isotope labeling and quantitative liquid chromatography/mass spectrometry
Abstract
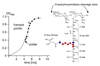
The molecular details of the biosynthesis and resulting architecture of the bacterial cell wall remain unclear but are essential to understanding the activity of glycopeptide antibiotics, the recognition of pathogens by hosts, and the processes of bacterial growth and division. Here we report a new strategy to elucidate bacterial cell-wall architecture based on time-dependent isotope labeling of bacterial cells quantified by liquid chromatography/accurate mass measurement mass spectrometry. The results allow us to track the fate of cell-wall precursors (which contain the vancomycin-binding site) in Enterococcus faecium, a leading antibiotic-resistant pathogen. By comparing isotopic enrichments of postinsertionally modified cell-wall precursors, we find that tripeptides and species without aspartic acid/asparagine (Asp/Asn, Asx) bridges are specific to mature cell wall. Additionally, we find that the sequence of cell-wall maturation varies throughout a cell cycle. We suggest that actively dividing E. faecium cells have three zones of unique peptidoglycan processing. Our results reveal new organizational characteristics of the bacterial cell wall that are important to understanding tertiary structure and designing novel drugs for antibiotic-resistant pathogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Aumeran C, Baud O, Lesens O, Delmas J, Souweine B, Traore O. Eur J Clin Microbiol Infect Dis. 2008 - PubMed
-
- Libisch B, Lepsanovic Z, Top J, Muzslay M, Konkoly-Thege M, Gacs M, Balogh B, Fuzi M, Willems RJ. Scand J Infect Dis. 2008:1–7. - PubMed
-
- Mainardi JL, Villet R, Bugg TD, Mayer C, Arthur M. FEMS Microbiol Rev. 2008;32:386–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

