Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction
- PMID: 19282289
- PMCID: PMC2685661
- DOI: 10.1074/jbc.M806486200
Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction
Abstract
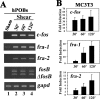
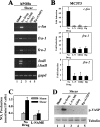
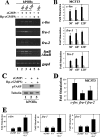
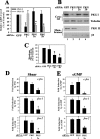
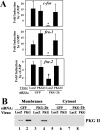
Continuous bone remodeling in response to mechanical loading is critical for skeletal integrity, and interstitial fluid flow is an important stimulus for osteoblast/osteocyte growth and differentiation. However, the biochemical signals mediating osteoblast anabolic responses to mechanical stimulation are incompletely understood. In primary human osteoblasts and murine MC3T3-E1 cells, we found that fluid shear stress induced rapid expression of c-fos, fra-1, fra-2, and fosB/DeltafosB mRNAs; these genes encode transcriptional regulators that maintain skeletal integrity. Fluid shear stress increased osteoblast nitric oxide (NO) synthesis, leading to activation of cGMP-dependent protein kinase (PKG). Pharmacological inhibition of the NO/cGMP/PKG signaling pathway blocked shear-induced expression of all four fos family genes. Induction of these genes required signaling through MEK/Erk, and Erk activation was NO/cGMP/PKG-dependent. Treating cells with a membrane-permeable cGMP analog partly mimicked the effects of fluid shear stress on Erk activity and fos family gene expression. In cells transfected with small interfering RNAs (siRNA) specific for membrane-bound PKG II, shear- and cGMP-induced Erk activation and fos family gene expression was nearly abolished and could be restored by transducing cells with a virus encoding an siRNA-resistant form of PKG II; in contrast, siRNA-mediated repression of the more abundant cytosolic PKG I isoform was without effect. Thus, we report a novel function for PKG II in osteoblast mechanotransduction, and we propose a model whereby NO/cGMP/PKG II-mediated Erk activation and induction of c-fos, fra-1, fra-2, and fosB/DeltafosB play a key role in the osteoblast anabolic response to mechanical stimulation.
Figures

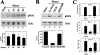



References
-
- Hughes-Fulford, M. (2004) Sci. STKE 2004, RE12 - PubMed
-
- Ehrlich, P. J., and Lanyon, L. E. (2002) Osteoporos. Int. 13 688–700 - PubMed
-
- Orr, A. W., Helmke, B. P., Blackman, B. R., and Schwartz, M. A. (2006) Dev. Cell 10 11–20 - PubMed
-
- Hillsley, M. V., and Frangos, J. A. (1994) Biotechnol. Bioeng. 43 573–581 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

