Mechanisms of protein kinase C signaling in the modulation of 3',5'-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells
- PMID: 19282384
- PMCID: PMC2703526
- DOI: 10.1210/en.2008-1668
Mechanisms of protein kinase C signaling in the modulation of 3',5'-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells
Abstract
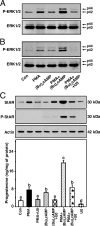
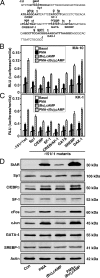
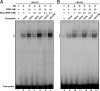
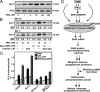
The protein kinase C (PKC) signaling pathway plays integral roles in the expression of the steroidogenic acute regulatory (StAR) protein that regulates steroid biosynthesis in steroidogenic cells. PKC can modulate the activity of cAMP/protein kinase A signaling involved in steroidogenesis; however, its mechanism remains obscure. In the present study, we demonstrate that activation of the PKC pathway, by phorbol 12-myristate 13-acetate (PMA), was capable of potentiating dibutyryl cAMP [(Bu)(2)cAMP]-stimulated StAR expression, StAR phosphorylation, and progesterone synthesis in both mouse Leydig (MA-10) and granulosa (KK-1) tumor cells. The steroidogenic potential of PMA and (Bu)(2)cAMP was linked with phosphorylation of ERK 1/2; however, inhibition of the latter demonstrated varying effects on steroidogenesis. Transcriptional activation of the StAR gene by PMA and (Bu)(2)cAMP was influenced by several factors, its up-regulation being dependent on phosphorylation of the cAMP response element binding protein (CREB). An oligonucleotide probe containing a CREB/activating transcription factor binding region in the StAR promoter was found to bind nuclear proteins in PMA and (Bu)(2)cAMP-treated MA-10 and KK-1 cells. Chromatin immunoprecipitation studies revealed that the induction of phosphorylated CREB was tightly correlated with in vivo protein-DNA interactions and recruitment of CREB binding protein to the StAR promoter. Ectopic expression of CREB binding protein enhanced CREB-mediated transcription of the StAR gene, an event that was markedly repressed by the adenovirus E1A oncoprotein. Further studies demonstrated that the activation of StAR expression and steroid synthesis by PMA and (Bu)(2)cAMP was associated with expression of the nuclear receptor Nur77, indicating its essential role in hormone-regulated steroidogenesis. Collectively, these findings provide insight into the mechanisms by which PKC modulates cAMP/protein kinase A responsiveness involved in regulating the steroidogenic response in mouse gonadal cells.
Figures


Similar articles
-
Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling.J Endocrinol. 2007 Apr;193(1):53-63. doi: 10.1677/JOE-06-0201. J Endocrinol. 2007. PMID: 17400803
-
The involvement of specific PKC isoenzymes in phorbol ester-mediated regulation of steroidogenic acute regulatory protein expression and steroid synthesis in mouse Leydig cells.Endocrinology. 2011 Jan;152(1):313-25. doi: 10.1210/en.2010-0874. Epub 2010 Nov 3. Endocrinology. 2011. PMID: 21047949 Free PMC article.
-
Role of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 in protein kinase A- and protein kinase C-mediated regulation of the steroidogenic acute regulatory protein expression in mouse Leydig tumor cells: mechanism of action.Endocrinology. 2009 Jan;150(1):187-99. doi: 10.1210/en.2008-0368. Epub 2008 Sep 11. Endocrinology. 2009. PMID: 18787026 Free PMC article.
-
Regulation of somatostatin gene transcription by cyclic adenosine monophosphate.Metabolism. 1996 Aug;45(8 Suppl 1):4-7. doi: 10.1016/s0026-0495(96)90068-2. Metabolism. 1996. PMID: 8769368 Review.
-
Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene.Mol Cell Endocrinol. 2009 Apr 10;302(1):1-11. doi: 10.1016/j.mce.2008.12.009. Epub 2008 Dec 25. Mol Cell Endocrinol. 2009. PMID: 19150388 Free PMC article. Review.
Cited by
-
Up-regulation of steroid biosynthesis by retinoid signaling: Implications for aging.Mech Ageing Dev. 2015 Sep;150:74-82. doi: 10.1016/j.mad.2015.08.007. Epub 2015 Aug 21. Mech Ageing Dev. 2015. PMID: 26303142 Free PMC article.
-
NR4A1 (Nur77) mediates thyrotropin-releasing hormone-induced stimulation of transcription of the thyrotropin β gene: analysis of TRH knockout mice.PLoS One. 2012;7(7):e40437. doi: 10.1371/journal.pone.0040437. Epub 2012 Jul 9. PLoS One. 2012. PMID: 22792320 Free PMC article.
-
Basic Leucine Zipper Transcription Factors as Important Regulators of Leydig Cells' Functions.Int J Mol Sci. 2022 Oct 25;23(21):12887. doi: 10.3390/ijms232112887. Int J Mol Sci. 2022. PMID: 36361676 Free PMC article. Review.
-
Growth Hormone and Insulin-Like Growth Factor Action in Reproductive Tissues.Front Endocrinol (Lausanne). 2019 Nov 12;10:777. doi: 10.3389/fendo.2019.00777. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31781044 Free PMC article. Review.
-
Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells.Mol Endocrinol. 2011 May;25(5):885-93. doi: 10.1210/me.2010-0489. Epub 2011 Feb 17. Mol Endocrinol. 2011. PMID: 21330403 Free PMC article.
References
-
- Clark BJ, Wells J, King SR, Stocco DM 1994 The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 - PubMed
-
- Lin D, Sugawara T, Strauss 3rd JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL 1995 Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 - PubMed
-
- Stocco DM, Clark BJ 1996 Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244 - PubMed
-
- Christenson LK, Strauss 3rd JF 2000 Steroidogenic acute regulatory protein (StAR) and the intramitochondrial translocation of cholesterol. Biochim Biophys Acta 1529:175–187 - PubMed
-
- Manna PR, Stocco DM 2005 Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord 5:93–108 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

