Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects
- PMID: 19282464
- PMCID: PMC2656275
- DOI: 10.1182/blood-2009-05-155689
Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects
Abstract
High levels of granulocyte/macrophage-colony-stimulating factor (GM-CSF) autoantibodies are thought to cause pulmonary alveolar proteinosis (PAP), a rare syndrome characterized by myeloid dysfunction resulting in pulmonary surfactant accumulation and respiratory failure. Paradoxically, GM-CSF autoantibodies have been reported to occur rarely in healthy people and routinely in pharmaceutical intravenous immunoglobulin (IVIG) purified from serum pooled from healthy subjects. These findings suggest that either GM-CSF autoantibodies are normally present in healthy people at low levels that are difficult to detect or that serum pooled for IVIG purification may include asymptomatic persons with high levels of GM-CSF autoantibodies. Using several experimental approaches, GM-CSF autoantibodies were detected in all healthy subjects evaluated (n = 72) at low levels sufficient to rheostatically regulate multiple myeloid functions. Serum GM-CSF was more abundant than previously reported, but more than 99% was bound and neutralized by GM-CSF autoantibody. The critical threshold of GM-CSF autoantibodies associated with the development of PAP was determined. Results demonstrate that free serum GM-CSF is tightly maintained at low levels, identify a novel potential mechanism of innate immune regulation, help define the therapeutic window for potential clinical use of GM-CSF autoantibodies to treat inflammatory and autoimmune diseases, and have implications for the pathogenesis of PAP.
Figures
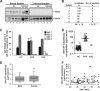


 ) or patients with PAP (■) and incubated with fresh whole blood at various concentrations. Each bar represents the results of 3 separate determinations. *Significant decrease (P < .001) from baseline determined in the absence of GM-CSF autoantibody. (F) Specificity of purified GM-CSF autoantibody. Neutrophils were incubated in the presence of GM-CSF (10 ng/mL) or IL-8 (100 ng/mL) and in the absence or presence of 1 μg GM-CSF autoantibody or control IgG as indicated. Data represent the level of CD11b in stimulated cells—the level in unstimulated cells. GM-CSF autoantibodies markedly inhibited the GM-CSF–stimulated (■), but resulted in levels of inhibition by IL-8 (
) or patients with PAP (■) and incubated with fresh whole blood at various concentrations. Each bar represents the results of 3 separate determinations. *Significant decrease (P < .001) from baseline determined in the absence of GM-CSF autoantibody. (F) Specificity of purified GM-CSF autoantibody. Neutrophils were incubated in the presence of GM-CSF (10 ng/mL) or IL-8 (100 ng/mL) and in the absence or presence of 1 μg GM-CSF autoantibody or control IgG as indicated. Data represent the level of CD11b in stimulated cells—the level in unstimulated cells. GM-CSF autoantibodies markedly inhibited the GM-CSF–stimulated (■), but resulted in levels of inhibition by IL-8 ( ) that were significantly lower and similar to control (IgG, □). *A significant difference (P < .001) compared with inhibition of the GM-CSF–stimulated increase by GM-CSF autoantibody (■). (G) The CD11b stimulation index was measured in fresh blood from healthy control subjects (○), patients with PAP with active disease (◇), or patients with PAP in clinical remission of the lung disease (♦). The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-24 μg/mL, ▨). Each symbol represents the results of triplicate determinations for one subject. The median (IQR) free serum GM-CSF level in healthy subjects was 0.00 (0.00-0.390) pg/mL and did not correlate with the CD11b stimulation index (P > .05), whereas the median (IQR) GM-CSF autoantibody level (0.90 [0.58-1.19] μg/mL) correlated with CD11b stimulation index (R2 = 0.46, P = .03) (Spearman rank order correlation).
) that were significantly lower and similar to control (IgG, □). *A significant difference (P < .001) compared with inhibition of the GM-CSF–stimulated increase by GM-CSF autoantibody (■). (G) The CD11b stimulation index was measured in fresh blood from healthy control subjects (○), patients with PAP with active disease (◇), or patients with PAP in clinical remission of the lung disease (♦). The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-24 μg/mL, ▨). Each symbol represents the results of triplicate determinations for one subject. The median (IQR) free serum GM-CSF level in healthy subjects was 0.00 (0.00-0.390) pg/mL and did not correlate with the CD11b stimulation index (P > .05), whereas the median (IQR) GM-CSF autoantibody level (0.90 [0.58-1.19] μg/mL) correlated with CD11b stimulation index (R2 = 0.46, P = .03) (Spearman rank order correlation).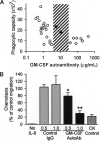
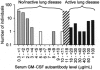
 , n = 72), patients with PAP with active lung disease (■, n = 21), and patients with PAP in clinical remission of the lung disease (□, n = 2). Clinical remission was defined as formerly diagnosed patients with PAP who were currently presenting no respiratory insufficiency and had normal chest X-ray images. The range of serum GM-CSF autoantibody levels separating subjects with no or active lung disease from those without active disease is indicated (10.4-19 μg/mL, ▨).
, n = 72), patients with PAP with active lung disease (■, n = 21), and patients with PAP in clinical remission of the lung disease (□, n = 2). Clinical remission was defined as formerly diagnosed patients with PAP who were currently presenting no respiratory insufficiency and had normal chest X-ray images. The range of serum GM-CSF autoantibody levels separating subjects with no or active lung disease from those without active disease is indicated (10.4-19 μg/mL, ▨).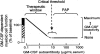
Comment in
-
Activated cryptic granulocyte-macrophage colony-stimulating factor autoantibodies in intravenous immunoglobulin preparations.Blood. 2010 Jan 14;115(2):431. doi: 10.1182/blood-2009-08-240309. Blood. 2010. PMID: 20075173 No abstract available.
-
Are neutralizing anti-GM-CSF autoantibodies present in all healthy persons?Blood. 2010 Jan 14;115(2):433-4. doi: 10.1182/blood-2009-08-241018. Blood. 2010. PMID: 20075174 No abstract available.
References
-
- Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU. 1. Immunity. 2001;15:557–567. - PubMed
-
- Hamilton JA, Anderson GP. GM-CSF Biology. Growth Factors. 2004;22:225–231. - PubMed
-
- Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. - PubMed
-
- Trapnell BC, Whitsett JA, Nakata K. Pulmonary Alveolar Proteinosis. N Engl J Med. 2003;349:2527–2539. - PubMed
-
- Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

