Braf(V600E) cooperates with Pten loss to induce metastatic melanoma
- PMID: 19282848
- PMCID: PMC2705918
- DOI: 10.1038/ng.356
Braf(V600E) cooperates with Pten loss to induce metastatic melanoma
Abstract
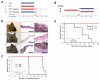
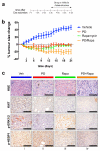
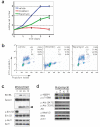
Mutational activation of BRAF is the earliest and most common genetic alteration in human melanoma. To build a model of human melanoma, we generated mice with conditional melanocyte-specific expression of BRaf(V600E). Upon induction of BRaf(V600E) expression, mice developed benign melanocytic hyperplasias that failed to progress to melanoma over 15-20 months. By contrast, expression of BRaf(V600E) combined with Pten tumor suppressor gene silencing elicited development of melanoma with 100% penetrance, short latency and with metastases observed in lymph nodes and lungs. Melanoma was prevented by inhibitors of mTorc1 (rapamycin) or MEK1/2 (PD325901) but, upon cessation of drug administration, mice developed melanoma, indicating the presence of long-lived melanoma-initiating cells in this system. Notably, combined treatment with rapamycin and PD325901 led to shrinkage of established melanomas. These mice, engineered with a common genetic profile to human melanoma, provide a system to study melanoma's cardinal feature of metastasis and for preclinical evaluation of agents designed to prevent or treat metastatic disease.
Figures




References
-
- Chin L, Merlino G, DePinho RA. Malignant melanoma: modern black plague and genetic black box. Genes Dev. 1998;12:3467–81. - PubMed
-
- Gray-Schopfer VC, da Rocha Dias S, Marais R. The role of B-RAF in melanoma. Cancer Metastasis Rev. 2005;24:165–83. - PubMed
-
- Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer. 2003;3:559–70. - PubMed
-
- Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
-
- Garraway LA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

