Trophic macrophages in development and disease
- PMID: 19282852
- PMCID: PMC3648866
- DOI: 10.1038/nri2528
Trophic macrophages in development and disease
Abstract
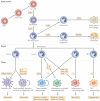
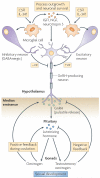
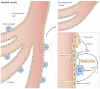
Specialized phagocytes are found in the most primitive multicellular organisms. Their roles in homeostasis and in distinguishing self from non-self have evolved with the complexity of organisms and their immune systems. Equally important, but often overlooked, are the roles of macrophages in tissue development. As discussed in this Review, these include functions in branching morphogenesis, neuronal patterning, angiogenesis, bone morphogenesis and the generation of adipose tissue. In each case, macrophage depletion impairs the formation of the tissue and compromises its function. I argue that in several diseases, the unrestrained acquisition of these developmental macrophage functions exacerbates pathology. For example, macrophages enhance tumour progression and metastasis by affecting tumour-cell migration and invasion, as well as angiogenesis.
Figures


References
-
- Tauber AI. Metchnikoff and the phagocytosis theory. Nature Rev. Mol. Cell Biol. 2003;4:897–901. - PubMed
-
- Kawai Y, Smedsrod B, Elvevold K, Wake K. Uptake of lithium carmine by sinusoidal endothelial and Kupffer cells of the rat liver: new insights into the classical vital staining and the reticulo-endothelial system. Cell Tissue Res. 1998;292:395–410. - PubMed
-
- Banchereau J, et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. - PubMed
-
- Hume DA. Macrophages as APC and the dendritic cell myth. J. Immunol. 2008;181:5829–5835. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

