Escherichia coli MazF leads to the simultaneous selective synthesis of both "death proteins" and "survival proteins"
- PMID: 19282968
- PMCID: PMC2646832
- DOI: 10.1371/journal.pgen.1000390
Escherichia coli MazF leads to the simultaneous selective synthesis of both "death proteins" and "survival proteins"
Abstract
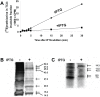
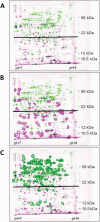
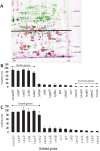
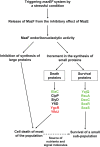
The Escherichia coli mazEF module is one of the most thoroughly studied toxin-antitoxin systems. mazF encodes a stable toxin, MazF, and mazE encodes a labile antitoxin, MazE, which prevents the lethal effect of MazF. MazF is an endoribonuclease that leads to the inhibition of protein synthesis by cleaving mRNAs at ACA sequences. Here, using 2D-gels, we show that in E. coli, although MazF induction leads to the inhibition of the synthesis of most proteins, the synthesis of an exclusive group of proteins, mostly smaller than about 20 kDa, is still permitted. We identified some of those small proteins by mass spectrometry. By deleting the genes encoding those proteins from the E. coli chromosome, we showed that they were required for the death of most of the cellular population. Under the same experimental conditions, which induce mazEF-mediated cell death, other such proteins were found to be required for the survival of a small sub-population of cells. Thus, MazF appears to be a regulator that induces downstream pathways leading to death of most of the population and the continued survival of a small sub-population, which will likely become the nucleus of a new population when growth conditions become less stressful.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
Life, death, differentiation, and the multicellularity of bacteria.PLoS Genet. 2009 Mar;5(3):e1000418. doi: 10.1371/journal.pgen.1000418. Epub 2009 Mar 13. PLoS Genet. 2009. PMID: 19282973 Free PMC article. Review. No abstract available.
References
-
- Engelberg-Kulka H, Sat B, Hazan R. Bacterial programmed cell death and antibiotics. ASM News. 2002;67:617–625.
-
- Engelberg-Kulka H, Sat B, Reches M, Amitai S, Hazan R. Bacterial programmed cell death systems as targets for antibiotics. Trends Microbiol. 2004;12:66–71. - PubMed
-
- Hayes F. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science. 2003;301:1496–1499. - PubMed
-
- Mittenhuber G. Occurrence of mazEF-like antitoxin/toxin systems in bacteria. J Mol Microbiol Biotechnol. 1999;1:295–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

