Caspar controls resistance to Plasmodium falciparum in diverse anopheline species
- PMID: 19282971
- PMCID: PMC2647737
- DOI: 10.1371/journal.ppat.1000335
Caspar controls resistance to Plasmodium falciparum in diverse anopheline species
Abstract
Immune responses mounted by the malaria vector Anopheles gambiae are largely regulated by the Toll and Imd (immune deficiency) pathways via the NF-kappaB transcription factors Rel1 and Rel2, which are controlled by the negative regulators Cactus and Caspar, respectively. Rel1- and Rel2-dependent transcription in A. gambiae has been shown to be particularly critical to the mosquito's ability to manage infection with the rodent malaria parasite Plasmodium berghei. Using RNA interference to deplete the negative regulators of these pathways, we found that Rel2 controls resistance of A. gambiae to the human malaria parasite Plasmodium falciparum, whereas Rel 1 activation reduced infection levels. The universal relevance of this defense system across Anopheles species was established by showing that caspar silencing also prevents the development of P. falciparum in the major malaria vectors of Asia and South America, A. stephensi and A. albimanus, respectively. Parallel studies suggest that while Imd pathway activation is most effective against P. falciparum, the Toll pathway is most efficient against P. berghei, highlighting a significant discrepancy between the human pathogen and its rodent model. High throughput gene expression analyses identified a plethora of genes regulated by the activation of the two Rel factors and revealed that the Toll pathway played a more diverse role in mosquito biology than the Imd pathway, which was more immunity-specific. Further analyses of key anti-Plasmodium factors suggest they may be responsible for the Imd pathway-mediated resistance phenotype. Additionally, we found that the fitness cost caused by Rel2 activation through caspar gene silencing was undetectable in sugar-fed, blood-fed, and P. falciparum-infected female A. gambiae, while activation of the Toll pathway's Rel1 had a major impact. This study describes for the first time a single gene that influences an immune mechanism that is able to abort development of P. falciparum in Anopheline species. Further, this study addresses aspects of the molecular, evolutionary, and physiological consequences of the observed phenotype. These findings have implications for malaria control since broad-spectrum immune activation in diverse anopheline species offers a viable and strategic approach to develop novel malaria control methods worldwide.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



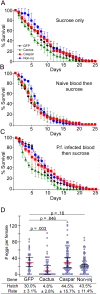
References
-
- Meister S, Koutsos AC, Christophides GK. The Plasmodium parasite—a ‘new’ challenge for insect innate immunity. Int J Parasitol. 2004;34:1473–82. - PubMed
-
- Michel K, Kafatos FC. Mosquito immunity against Plasmodium. Ins Biochem Mol Biol. 2005;35:677–689. - PubMed
-
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, et al. Immunity-related genes and gene-families in Anopheles gambiae. Science. 2002;298:159–165. - PubMed
-
- Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

