The human vaginal bacterial biota and bacterial vaginosis
- PMID: 19282975
- PMCID: PMC2648628
- DOI: 10.1155/2008/750479
The human vaginal bacterial biota and bacterial vaginosis
Abstract
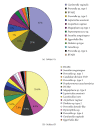
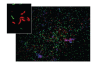
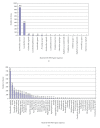
The bacterial biota of the human vagina can have a profound impact on the health of women and their neonates. Changes in the vaginal microbiota have been associated with several adverse health outcomes including premature birth, pelvic inflammatory disease, and acquisition of HIV infection. Cultivation-independent molecular methods have provided new insights regarding bacterial diversity in this important niche, particularly in women with the common condition bacterial vaginosis (BV). PCR methods have shown that women with BV have complex communities of vaginal bacteria that include many fastidious species, particularly from the phyla Bacteroidetes and Actinobacteria. Healthy women are mostly colonized with lactobacilli such as Lactobacillus crispatus, Lactobacillus jensenii, and Lactobacillus iners, though a variety of other bacteria may be present. The microbiology of BV is heterogeneous. The presence of Gardnerella vaginalis and Atopobium vaginae coating the vaginal epithelium in some subjects with BV suggests that biofilms may contribute to this condition.
Figures




References
-
- Evans AS. Causation and Disease. New York, NY, USA: Plenum; 1993.
-
- Vallor AC, Antonio MAD, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. The Journal of Infectious Diseases. 2001;184(11):1431–1436. - PubMed
-
- Hillier SL, Krohn MA, Nugent RP, Gibbs RS. Characteristics of three vaginal flora patterns assessed by Gram stain among pregnant women. Vaginal Infections and Prematurity Study Group. American Journal of Obstetrics and Gynecology. 1992;166(3):938–944. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

