A GRFa2/Prop1/stem (GPS) cell niche in the pituitary
- PMID: 19283075
- PMCID: PMC2654029
- DOI: 10.1371/journal.pone.0004815
A GRFa2/Prop1/stem (GPS) cell niche in the pituitary
Abstract
Background: The adult endocrine pituitary is known to host several hormone-producing cells regulating major physiological processes during life. Some candidates to progenitor/stem cells have been proposed. However, not much is known about pituitary cell renewal throughout life and its homeostatic regulation during specific physiological changes, such as puberty or pregnancy, or in pathological conditions such as tumor development.
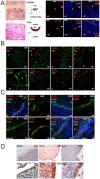
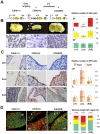
Principal findings: We have identified in rodents and humans a niche of non-endocrine cells characterized by the expression of GFRa2, a Ret co-receptor for Neurturin. These cells also express b-Catenin and E-cadherin in an oriented manner suggesting a planar polarity organization for the niche. In addition, cells in the niche uniquely express the pituitary-specific transcription factor Prop1, as well as known progenitor/stem markers such as Sox2, Sox9 and Oct4. Half of these GPS (GFRa2/Prop1/Stem) cells express S-100 whereas surrounding elongated cells in contact with GPS cells express Vimentin. GFRa2+-cells form non-endocrine spheroids in culture. These spheroids can be differentiated to hormone-producing cells or neurons outlining the neuroectoderm potential of these progenitors. In vivo, GPSs cells display slow proliferation after birth, retain BrdU label and show long telomeres in its nuclei, indicating progenitor/stem cell properties in vivo.
Significance: Our results suggest the presence in the adult pituitary of a specific niche of cells characterized by the expression of GFRa2, the pituitary-specific protein Prop1 and stem cell markers. These GPS cells are able to produce different hormone-producing and neuron-like cells and they may therefore contribute to postnatal pituitary homeostasis. Indeed, the relative abundance of GPS numbers is altered in Cdk4-deficient mice, a model of hypopituitarism induced by the lack of this cyclin-dependent kinase. Thus, GPS cells may display functional relevance in the physiological expansion of the pituitary gland throughout life as well as protection from pituitary disease.
Conflict of interest statement
Figures




References
-
- Vankelecom H. Non-hormonal cell types in the pituitary candidating for stem cell. Semin Cell Dev Biol. 2007;18(4):559–570. - PubMed
-
- Allaerts W, Vankelecom H. History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol. 2005;153(1):1–12. - PubMed
-
- Horvath E, Kovacs K. Folliculo-stellate cells of the human pituitary: A type of adult stem cell? Ultrastruct Pathol. 2002;26(4):219–228. - PubMed
-
- Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: Signaling and transcriptional networks. Physiol Rev. 2007;87(3):933–963. - PubMed
-
- Vankelecom H. Stem cells in the postnatal pituitary? Neuroendocrinology. 2007;85(2):110–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

