Generation of transgenic mice
- PMID: 19283729
- PMCID: PMC3119260
- DOI: 10.1002/0471143030.cb1911s42
Generation of transgenic mice
Abstract
This unit describes detailed step-by-step protocols, reagents, and equipment required for successful generation of transgenic mice using pronuclear injection. The experimental methods and practical tips given here will help guide beginners in understanding what is required and what to avoid in these standard protocols for efficiently generating transgenic mice.
Copyright 2009 by John Wiley & Sons, Inc.
Figures
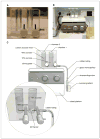



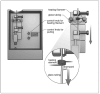




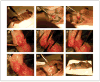
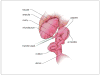
References
Literature Cited
-
- Brown T. Southern Blotting. Curr Protoc Molec Biol. 1993;21:2.9.1–2.9.20.
-
- Conner DA. Transgenic mouse production by zygote injection. Curr Protoc Molec Biol. 2004;68:23.9.1–23.9.23. - PubMed
-
- Donovan J, Brown P. Parenteral injections. Curr Protoc Immunol. 2006a;73:1.6.1–1.6.10. - PubMed
-
- Donovan J, Brown P. Euthanasia. Curr Protoc Immunol. 2006b;73:1.8.1–1.8.4. - PubMed
Key References
-
- Brown GAJ, Corbin TJ. Transgenesis in the Mouse: Oocyte injection. In: Clarke AR, editor. Transgenesis Techniques. Methods in Molecular Biology. Vol. 180. Humana Press; Totowa, New Jersey: 2002. pp. 39–70. This chapter contains very useful information for the production of transgenic mice, especially for setting up a new transgenic facility.
-
-
Conner, 2004. See above.
The most current information and methodologies for the generation of transgenic mice are found in this protocol.
-
-
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A laboratory Manual. 3rd. Cold Spring Laboratory Press, Cold Spring Harbor; New York: 2003. A comprehensive guide for generating genetically altered mice along with excellent diagrams and illustrations.
-
- Overbeek PA. Factors Affecting Transgenic Animal Production. In: Pinkert CA, editor. Transgenic Animal Technology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 72–109. A detailed description of different strains of mice, excellent husbandry practices, and transgenic phenomenology are discussed in this chapter.
-
- Pinkert CA. Introduction to Transgenic Animals. In: Pinkert CA, editor. Transgenic Animal Technology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 3–11. A thorough and complete history of the development of transgenic technology, as well as a compendium for practical applications of transgenesis via pronuclear injections. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

