The role of tau in neurodegeneration
- PMID: 19284597
- PMCID: PMC2663562
- DOI: 10.1186/1750-1326-4-13
The role of tau in neurodegeneration
Abstract
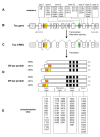
Since the identification of tau as the main component of neurofibrillary tangles in Alzheimer's disease and related tauopathies, and the discovery that mutations in the tau gene cause frontotemporal dementia, much effort has been directed towards determining how the aggregation of tau into fibrillar inclusions causes neuronal death. As evidence emerges that tau-mediated neuronal death can occur even in the absence of tangle formation, a growing number of studies are focusing on understanding how abnormalities in tau (e.g. aberrant phosphorylation, glycosylation or truncation) confer toxicity. Though data obtained from experimental models of tauopathies strongly support the involvement of pathologically modified tau and tau aggregates in neurodegeneration, the exact neurotoxic species remain unclear, as do the mechanism(s) by which they cause neuronal death. Nonetheless, it is believed that tau-mediated neurodegeneration is likely to result from a combination of toxic gains of function as well as from the loss of normal tau function. To truly appreciate the detrimental consequences of aberrant tau function, a better understanding of all functions carried out by tau, including but not limited to the role of tau in microtubule assembly and stabilization, is required. This review will summarize what is currently known regarding the involvement of tau in the initiation and development of neurodegeneration in tauopathies, and will also highlight some of the remaining questions in need of further investigation.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

