Connexins: a myriad of functions extending beyond assembly of gap junction channels
- PMID: 19284610
- PMCID: PMC2660342
- DOI: 10.1186/1478-811X-7-4
Connexins: a myriad of functions extending beyond assembly of gap junction channels
Abstract
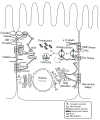
Connexins constitute a large family of trans-membrane proteins that allow intercellular communication and the transfer of ions and small signaling molecules between cells. Recent studies have revealed complex translational and post-translational mechanisms that regulate connexin synthesis, maturation, membrane transport and degradation that in turn modulate gap junction intercellular communication. With the growing myriad of connexin interacting proteins, including cytoskeletal elements, junctional proteins, and enzymes, gap junctions are now perceived, not only as channels between neighboring cells, but as signaling complexes that regulate cell function and transformation. Connexins have also been shown to form functional hemichannels and have roles altogether independent of channel functions, where they exert their effects on proliferation and other aspects of life and death of the cell through mostly-undefined mechanisms. This review provides an updated overview of current knowledge of connexins and their interacting proteins, and it describes connexin modulation in disease and tumorigenesis.
Figures
References
-
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. - PubMed
-
- Carraway KL, Ramsauer VP, Carraway CA. Glycoprotein contributions to mammary gland and mammary tumor structure and function: Roles of adherens junctions, ErbBs and membrane MUCs. J Cell Biochem. 2005;96:914–926. - PubMed
-
- Liu H, Radisky DC, Bissell MJ. Proliferation and polarity in breast cancer: Untying the gordian knot. Cell Cycle. 2005;4:646–649. - PubMed
-
- Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol. 2004;16:140–145. - PubMed
-
- Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Connexins and their channels in cell growth and cell death. Cell Signal. 2006;18:592–600. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

