Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells
- PMID: 19286133
- PMCID: PMC3142922
- DOI: 10.1016/j.chom.2009.02.005
Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells
Abstract
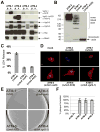
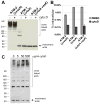
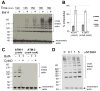
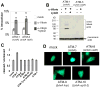
The type VI secretion system (T6SS) is a virulence mechanism common to several Gram-negative pathogens. In Vibrio cholerae, VgrG-1 is required for T6SS-dependent secretion. VgrG-1 is also secreted by T6SS and displays a C-terminal actin crosslinking domain (ACD). Using a heterologous reporter enzyme in place of the ACD, we show that the effector and secretion functions of VgrG-1 are genetically dissociable with the ACD being dispensable for secretion but required for T6SS-dependent phenotypes. Furthermore, internalization of bacteria is required for ACD translocation into phagocytic target cells. Inhibiting bacterial uptake abolishes actin crosslinking, while improving intracellular survival enhances it. Otherwise resistant nonphagocytic cells become susceptible to T6SS-mediated actin crosslinking when engineered to take up bacteria. Our results support a model for translocation of VgrG C-terminal effector domains into target cell cytosol by a process that requires trafficking of bacterial cells into an endocytic compartment where translocation is triggered by an unknown signal.
Figures


Comment in
-
Bacterial martyrdom: phagocytes disabled by type VI secretion after engulfing bacteria.Cell Host Microbe. 2009 Mar 19;5(3):213-4. doi: 10.1016/j.chom.2009.03.001. Cell Host Microbe. 2009. PMID: 19286128
References
-
- Abd H, Weintraub A, Sandstrom G. Intracellular survival and replication of Vibrio cholerae O139 in aquatic free-living amoebae. Environ Microbiol. 2005;7:1003–1008. - PubMed
-
- Barbieri JT, Riese MJ, Aktories K. Bacterial toxins that modify the actin cytoskeleton. Annu Rev Cell Dev Biol. 2002;18:315–344. - PubMed
-
- Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: a beginner’s guide. Curr Opin Microbiol. 2008;11:3–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

