Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states
- PMID: 19286473
- PMCID: PMC2757135
- DOI: 10.1016/j.bbalip.2009.03.001
Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states
Abstract
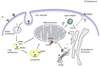
The transfer of cholesterol from the outer to the inner mitochondrial membrane is the rate-limiting step in hormone-induced steroid formation. To ensure that this step is achieved efficiently, free cholesterol must accumulate in excess at the outer mitochondrial membrane and then be transferred to the inner membrane. This is accomplished through a series of steps that involve various intracellular organelles, including lysosomes and lipid droplets, and proteins such as the translocator protein (18 kDa, TSPO) and steroidogenic acute regulatory (StAR) proteins. TSPO, previously known as the peripheral-type benzodiazepine receptor, is a high-affinity drug- and cholesterol-binding mitochondrial protein. StAR is a hormone-induced mitochondria-targeted protein that has been shown to initiate cholesterol transfer into mitochondria. Through the assistance of proteins such as the cAMP-dependent protein kinase regulatory subunit Ialpha (PKA-RIalpha) and the PKA-RIalpha- and TSPO-associated acyl-coenzyme A binding domain containing 3 (ACBD3) protein, PAP7, cholesterol is transferred to and docked at the outer mitochondrial membrane. The TSPO-dependent import of StAR into mitochondria, and the association of TSPO with the outer/inner mitochondrial membrane contact sites, drives the intramitochondrial cholesterol transfer and subsequent steroid formation. The focus of this review is on (i) the intracellular pathways and protein-protein interactions involved in cholesterol transport and steroid biosynthesis and (ii) the roles and interactions of these proteins in endocrine pathologies and neurological diseases where steroid synthesis plays a critical role.
Figures

References
-
- Hall PF, et al. Role of cytochromes P-450 in the biosynthesis of steroid hormones. Vitam. Horm. 1985;42:315–368. - PubMed
-
- Simpson ER, Waterman MR, et al. Regulation by ACTH of steroid hormone biosynthesis in the adrenal cortex. Can. J. Biochem. Cell Biol. 1983;61:692–707. - PubMed
-
- Garren LD, Gill GN, Masui H, Walton GM. On the mechanism of action of ACTH. Recent Prog. Horm. Res. 1971;27:433–478. - PubMed
-
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann PL, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27:402–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

