ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse
- PMID: 19286647
- PMCID: PMC2694331
- DOI: 10.1194/jlr.M900024-JLR200
ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse
Abstract
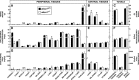
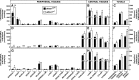
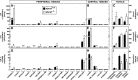
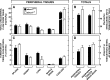
This study uses the mouse to explore the role of ABCA1 in the movement of this cholesterol from the peripheral organs to the endocrine glands for hormone synthesis and liver for excretion. The sterol pool in all peripheral organs was constant and equaled 2,218 and 2,269 mg/kg, respectively, in abca1(+/+) and abca1(-/-) mice. Flux of cholesterol from these tissues equaled the rate of synthesis plus the rate of LDL-cholesterol uptake and was 49.9 mg/day/kg in control animals and 62.0 mg/day/kg in abca1(-/-) mice. In the abca1(+/+) animals, this amount of cholesterol moved from HDL into the liver for excretion. In the abca1(-/-) mice, the cholesterol from the periphery also reached the liver but did not use HDL. Fecal excretion of cholesterol was just as high in abac1(-/-) mice (198 mg/day/kg) as in the abac1(+/+) animals (163 mg/day/kg), although the abac1(-/-) mice excreted relatively more neutral than acidic sterols. This study established that ABCA1 plays essentially no role in the turnover of cholesterol in peripheral organs or in the centripetal movement of this sterol to the endocrine glands, liver, and intestinal tract for excretion.
Figures



References
-
- Simons K., and E. Ikonen. 2000. How cells handle cholesterol. Science. 290 1721–1726. - PubMed
-
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1989. The plasma membrane. In Molecular Biology of the Cell. M. Robertson, editor. Garland Publishing, New York. 275–340.
-
- Dietschy J. M., T. Kita, K. E. Suckling, J. L. Goldstein, and M. S. Brown. 1983. Cholesterol synthesis in vivo and in vitro in the WHHL rabbit, an animal with defective low density lipoprotein receptors. J. Lipid Res. 24 469–480. - PubMed
-
- Quan G., C. Xie, J. M. Dietschy, and S. D. Turley. 2003. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res. Dev. Brain Res. 146 87–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

