Thioredoxins in redox maintenance and survival during oxidative stress of Bacteroides fragilis
- PMID: 19286811
- PMCID: PMC2687162
- DOI: 10.1128/JB.01665-08
Thioredoxins in redox maintenance and survival during oxidative stress of Bacteroides fragilis
Abstract
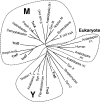
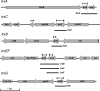
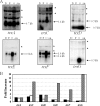
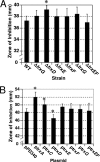
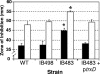
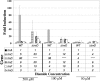
The anaerobe Bacteroides fragilis is a gram-negative, opportunistic pathogen that is highly aerotolerant and can persist in aerobic environments for extended periods. In this study, the six B. fragilis thioredoxins (Trxs) were investigated to determine their role during oxidative stress. Phylogenetic analyses of Trx protein sequences indicated that four of the six Trxs (TrxA, TrxC, TrxD, and TrxF) belong to the M-type Trx class but were associated with two different M-type lineages. TrxE and TrxG were most closely associated to Y-type Trxs found primarily in cyanobacteria. Single and multiple trx gene deletions were generated to determine functional differences between the Trxs. The trxA gene was essential, but no anaerobic growth defects were observed for any other single trx deletion or for the DeltatrxC DeltatrxD::cfxA DeltatrxE DeltatrxF DeltatrxG quintuple mutant. Regulation of the trx genes was linked to the oxidative stress response, and all were induced by aerobic conditions. The DeltatrxC DeltatrxE DeltatrxF DeltatrxG and the DeltatrxC DeltatrxD::cfxA DeltatrxE DeltatrxF DeltatrxG multiple deletion strains were impaired during growth in oxidized media, but single trx gene mutants did not have a phenotype in this assay. TrxD was protective during exposure to the thiol oxidant diamide, and expression of trxD was induced by diamide. Diamide-induced expression of trxC, trxE, and trxF increased significantly in a trxD mutant strain, suggesting that there is some capacity for compensation in this complex Trx system. These data provide insight into the role of individual Trxs in the B. fragilis oxidative stress response.
Figures

Similar articles
-
Thioredoxin reductase is essential for thiol/disulfide redox control and oxidative stress survival of the anaerobe Bacteroides fragilis.J Bacteriol. 2007 Nov;189(22):8015-23. doi: 10.1128/JB.00714-07. Epub 2007 Sep 14. J Bacteriol. 2007. PMID: 17873045 Free PMC article.
-
Two Lactococcus lactis thioredoxin paralogues play different roles in responses to arsenate and oxidative stress.Microbiology (Reading). 2015 Mar;161(Pt 3):528-38. doi: 10.1099/mic.0.000029. Epub 2015 Jan 6. Microbiology (Reading). 2015. PMID: 25564497
-
Distinct Roles of Shewanella oneidensis Thioredoxin in Regulation of Cellular Responses to Hydrogen and Organic Peroxides.Appl Environ Microbiol. 2019 Oct 16;85(21):e01700-19. doi: 10.1128/AEM.01700-19. Print 2019 Nov 1. Appl Environ Microbiol. 2019. PMID: 31444207 Free PMC article.
-
Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress.Free Radic Biol Med. 2019 Aug 20;140:28-35. doi: 10.1016/j.freeradbiomed.2019.03.003. Epub 2019 Mar 9. Free Radic Biol Med. 2019. PMID: 30862542 Review.
-
Thioredoxins and thioredoxin reductase in chloroplasts: A review.Gene. 2019 Jul 20;706:32-42. doi: 10.1016/j.gene.2019.04.041. Epub 2019 Apr 24. Gene. 2019. PMID: 31028868 Review.
Cited by
-
Effect of hyperbaric air on endotoxin from Bacteroides fragilis strains.Folia Microbiol (Praha). 2018 May;63(3):283-290. doi: 10.1007/s12223-017-0564-1. Epub 2017 Nov 13. Folia Microbiol (Praha). 2018. PMID: 29134546
-
Expression of Bacteroides fragilis hemolysins in vivo and role of HlyBA in an intra-abdominal infection model.Microbiologyopen. 2013 Apr;2(2):326-37. doi: 10.1002/mbo3.76. Epub 2013 Feb 26. Microbiologyopen. 2013. PMID: 23441096 Free PMC article.
-
Deciphering the Role of Multiple Thioredoxin Fold Proteins of Leptospirillum sp. in Oxidative Stress Tolerance.Int J Mol Sci. 2020 Mar 10;21(5):1880. doi: 10.3390/ijms21051880. Int J Mol Sci. 2020. PMID: 32164170 Free PMC article.
-
Analysis of Six tonB Gene Homologs in Bacteroides fragilis Revealed That tonB3 is Essential for Survival in Experimental Intestinal Colonization and Intra-Abdominal Infection.Infect Immun. 2022 Jan 25;90(1):e0046921. doi: 10.1128/IAI.00469-21. Epub 2021 Oct 18. Infect Immun. 2022. PMID: 34662212 Free PMC article.
-
Cold plasma pretreatment reinforces the lignocellulose-derived aldehyde inhibitors tolerance and bioethanol fermentability for Zymomonas mobilis.Biotechnol Biofuels Bioprod. 2023 Jun 15;16(1):102. doi: 10.1186/s13068-023-02354-8. Biotechnol Biofuels Bioprod. 2023. PMID: 37322470 Free PMC article.
References
-
- Arner, E. S., and A. Holmgren. 2000. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2676102-6109. - PubMed
-
- Baughn, A. D., and M. H. Malamy. 2004. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427441-444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

