Type II NADH dehydrogenase inhibitor 1-hydroxy-2-dodecyl-4(1H)quinolone leads to collapse of mitochondrial inner-membrane potential and ATP depletion in Toxoplasma gondii
- PMID: 19286986
- PMCID: PMC2698307
- DOI: 10.1128/EC.00381-08
Type II NADH dehydrogenase inhibitor 1-hydroxy-2-dodecyl-4(1H)quinolone leads to collapse of mitochondrial inner-membrane potential and ATP depletion in Toxoplasma gondii
Abstract
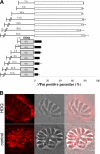
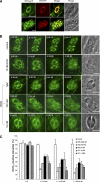
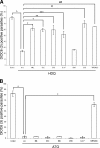
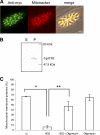
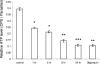
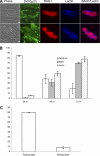
The apicomplexan parasite Toxoplasma gondii expresses type II NADH dehydrogenases (NDH2s) instead of canonical complex I at the inner mitochondrial membrane. These non-proton-pumping enzymes are considered to be promising drug targets due to their absence in mammalian cells. We recently showed by inhibition kinetics that T. gondii NDH2-I is a target of the quinolone-like compound 1-hydroxy-2-dodecyl-4(1H)quinolone (HDQ), which inhibits T. gondii replication in the nanomolar range. In this study, the cationic fluorescent probes Mitotracker and DiOC(6)(3) (3,3'-dihexyloxacarbocyanine iodine) were used to monitor the influence of HDQ on the mitochondrial inner membrane potential (Delta Psi m) in T. gondii. Real-time imaging revealed that nanomolar HDQ concentrations led to a Delta Psi m collapse within minutes, which is followed by severe ATP depletions of 30% after 1 h and 70% after 24 h. Delta Psi m depolarization was attenuated when substrates for other dehydrogenases that can donate electrons to ubiquinone were added to digitonin-permeabilized cells or when infected cultures were treated with the F(o)-ATPase inhibitor oligomycin. A prolonged treatment with sublethal concentrations of HDQ induced differentiation into bradyzoites. This dormant stage is likely to be less dependent on the Delta Psi m, since Delta Psi m-positive parasites were found at a significantly lower frequency in alkaline-pH-induced bradyzoites than in tachyzoites. Together, our studies reveal that oxidative phosphorylation is essential for maintaining the ATP level in the fast-growing tachyzoite stage and that HDQ interferes with this pathway by inhibiting the electron transport chain at the level of ubiquinone reduction.
Figures


References
-
- Bohne, W., U. Gross, D. J. Ferguson, and J. Heesemann. 1995. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol. Microbiol. 161221-1230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

