Linking conformation change to hemoglobin activation via chain-selective time-resolved resonance Raman spectroscopy of protoheme/mesoheme hybrids
- PMID: 19288145
- PMCID: PMC2880192
- DOI: 10.1007/s00775-009-0487-7
Linking conformation change to hemoglobin activation via chain-selective time-resolved resonance Raman spectroscopy of protoheme/mesoheme hybrids
Abstract
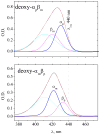
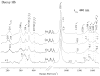
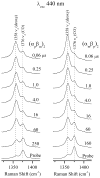
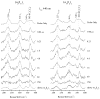
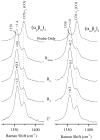
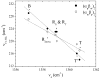
Time-resolved resonance Raman (RR) spectra are reported for hemoglobin (Hb) tetramers, in which the alpha and beta chains are selectively substituted with mesoheme. The Soret absorption band shift in mesoheme relative to protoheme permits chain-selective recording of heme RR spectra. The evolution of these spectra following HbCO photolysis shows that the geminate recombination rates and the yields are the same for the two chains, consistent with recent results on (15)N-heme isotopomer hybrids. The spectra also reveal systematic shifts in the deoxyheme nu (4) and nu (Fe-His) RR bands, which are anticorrelated. These shifts are resolved for the successive intermediates in the protein structure, which have previously been determined from time-resolved UV RR spectra. Both chains show Fe-His bond compression in the immediate photoproduct, which relaxes during the formation of the first intermediate, R(deoxy) (0.07 micros), in which the proximal F-helix is proposed to move away from the heme. Subsequently, the Fe-His bond weakens, more so for the alpha chains than for the beta chains. The weakening is gradual for the beta chains, but is abrupt for the alpha chains, coinciding with completion of the R-T quaternary transition, at 20 micros. Since the transition from fast- to slow-rebinding Hb also occurs at 20 micros, the drop in the alpha chain nu (Fe-His) supports the localization of ligation restraint to tension in the Fe-His bond, at least in the alpha chains. The mechanism is more complex in the beta chains.
Figures



Similar articles
-
Differential control of heme reactivity in alpha and beta subunits of hemoglobin: a combined Raman spectroscopic and computational study.J Am Chem Soc. 2014 Jul 23;136(29):10325-39. doi: 10.1021/ja503328a. Epub 2014 Jul 14. J Am Chem Soc. 2014. PMID: 24991732 Free PMC article.
-
Subunit-selective interrogation of CO recombination in carbonmonoxy hemoglobin by isotope-edited time-resolved resonance Raman spectroscopy.Biochemistry. 2009 Apr 14;48(14):3120-6. doi: 10.1021/bi802190f. Biochemistry. 2009. PMID: 19245215 Free PMC article.
-
A possible allosteric communication pathway identified through a resonance Raman study of four beta37 mutants of human hemoglobin A.Biochemistry. 1998 Mar 31;37(13):4346-57. doi: 10.1021/bi9708693. Biochemistry. 1998. PMID: 9521755
-
Structural heterogeneity of the Fe(2+)-N epsilon (HisF8) bond in various hemoglobin and myoglobin derivatives probed by the Raman-active iron histidine stretching mode.Biophys J. 1993 Oct;65(4):1470-85. doi: 10.1016/S0006-3495(93)81216-5. Biophys J. 1993. PMID: 8274641 Free PMC article.
-
Probing protein structure and dynamics with resonance Raman spectroscopy: cytochrome c peroxidase and hemoglobin.Biochemistry. 1990 May 15;29(19):4497-508. doi: 10.1021/bi00471a001. Biochemistry. 1990. PMID: 2164841 Review.
Cited by
-
Differential control of heme reactivity in alpha and beta subunits of hemoglobin: a combined Raman spectroscopic and computational study.J Am Chem Soc. 2014 Jul 23;136(29):10325-39. doi: 10.1021/ja503328a. Epub 2014 Jul 14. J Am Chem Soc. 2014. PMID: 24991732 Free PMC article.
-
Kinetic spectroscopy of heme hydration and ligand binding in myoglobin and isolated hemoglobin chains: an optical window into heme pocket water dynamics.Phys Chem Chem Phys. 2010 Sep 21;12(35):10270-8. doi: 10.1039/c003606b. Epub 2010 Jul 29. Phys Chem Chem Phys. 2010. PMID: 20668762 Free PMC article.
-
Quaternary structure controls ligand dynamics in soluble guanylate cyclase.J Biol Chem. 2012 Feb 24;287(9):6851-9. doi: 10.1074/jbc.M111.299297. Epub 2012 Jan 4. J Biol Chem. 2012. PMID: 22223482 Free PMC article.
-
Heme reactivity is uncoupled from quaternary structure in gel-encapsulated hemoglobin: a resonance Raman spectroscopic study.J Am Chem Soc. 2012 Feb 22;134(7):3461-71. doi: 10.1021/ja210126j. Epub 2012 Feb 9. J Am Chem Soc. 2012. PMID: 22263778 Free PMC article.
-
Role of Heme Pocket Water in Allosteric Regulation of Ligand Reactivity in Human Hemoglobin.Biochemistry. 2016 Jul 26;55(29):4005-17. doi: 10.1021/acs.biochem.6b00081. Epub 2016 Jul 13. Biochemistry. 2016. PMID: 27355904 Free PMC article.
References
-
- Perutz MF. Nature. 1970;228:726–734. - PubMed
-
- Baldwin J, Chothia C. J Mol Biol. 1979;129:175–220. - PubMed
-
- Monod J, Wyman J, Changeux JP. J Mol Biol. 1965;12:88–118. - PubMed
-
- Perutz MF. Mechanisms of Cooperativity and Allosteric Regulation in Proteins. Cambridge University Press; Cambridge: 1990. - PubMed
-
- Friedman JM, Scott TW, Stepnoski RA, Ikedasaito M, Yonetani T. J Biol Chem. 1983;258:564–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

