Increased accumulation of intraneuronal amyloid beta in HIV-infected patients
- PMID: 19288297
- PMCID: PMC3055557
- DOI: 10.1007/s11481-009-9152-8
Increased accumulation of intraneuronal amyloid beta in HIV-infected patients
Abstract
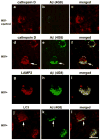
In recent years, human immunodeficiency virus (HIV)-infected patients under highly active anti-retroviral therapy (HAART) regimens have shown a markedly improved general clinical status; however, the prevalence of mild cognitive disorders has increased. We propose that increased longevity with HIV-mediated chronic inflammation combined with the secondary effects of HAART may increase the risk of early brain aging as shown by intraneuronal accumulation of abnormal protein aggregates like amyloid beta (Abeta), which might participate in worsening the neurodegenerative process and cognitive impairment in older patients with HIV. For this purpose, levels and distribution of Abeta immunoreactivity were analyzed in the frontal cortex of 43 patients with HIV (ages 38-60) and HIV- age-matched controls. Subcellular localization of the Abeta-immunoreactive material was analyzed by double labeling and confocal microscopy and by immunono-electron microscopy (EM). Compared to HIV- cases, in HIV+ cases, there was abundant intracellular Abeta immunostaining in pyramidal neurons and along axonal tracts. Cases with HIV encephalitis (HIVE) had higher levels of intraneuronal Abeta immunoreactivity compared to HIV+ cases with no HIVE. Moreover, levels of intracellular Abeta correlated with age in the group with HIVE. Double-labeling analysis showed that the Abeta-immunoreactive granules in the neurons co-localized with lysosomal markers such as cathepsin-D and LC3. Ultrastructural analysis by immuno-EM has confirmed that in these cases, intracellular Abeta was often found in structures displaying morphology similar to autophagosomes. These findings suggest that long-term survival with HIV might interfere with clearance of proteins such as Abeta and worsen neuronal damage and cognitive impairment in this population.
Figures

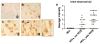

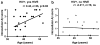

References
-
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. - DOI - PubMed
-
- An SF, Giometto B, Groves M, Miller RF, Beckett AA, Gray F, Tavolato B, Scaravilli F. Axonal damage revealed by accumulation of beta-APP in HIV-positive individuals without AIDS. J Neuropathol Exp Neurol. 1997;56:1262–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH045294/MH/NIMH NIH HHS/United States
- MH5974/MH/NIMH NIH HHS/United States
- DA12065/DA/NIDA NIH HHS/United States
- R21 MH076681/MH/NIMH NIH HHS/United States
- U01 MH83506/MH/NIMH NIH HHS/United States
- R01 MH079881/MH/NIMH NIH HHS/United States
- MH58164/MH/NIMH NIH HHS/United States
- R21 MH072529/MH/NIMH NIH HHS/United States
- MH79881/MH/NIMH NIH HHS/United States
- MH076681/MH/NIMH NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
- P01 DA012065/DA/NIDA NIH HHS/United States
- U01 MH083506/MH/NIMH NIH HHS/United States
- MH 62512/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

